1. Introduction
1.1. Serious mental illness, physical co-morbidity and premature mortality
Severe mental illnesses (SMI), defined as schizophrenia-spectrum disorders, bipolar disorder (BD) and major depressive disorder (MDD), are leading causes of years lived with global disability and are of considerable public health importance [Reference Whiteford, Ferrari, Degenhardt, Feigin and Vos1]. In addition to the impact of the mental health symptoms and reduced daily life functioning, people with SMI are at increased risk of premature mortality by between 10–20 years compared to age- and sex-matched controls [Reference Walker, McGee and Druss2–Reference Hayes, Miles, Walters, King and Osborn5]. While suicide accounts for a concerning portion of the early mortality [Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine, Charlson, Norman, Flaxman, Johns, Burstein, Murray, Vos, Whiteford and Degenhardt6, Reference Chesney, Goodwin and Fazel7], there is increasing recognition that physical disorders account for approximately 70% of these premature deaths [Reference Nielsen, Uggerby, Jensen and McGrath3, Reference Correll, Detraux, De Lepeleire and De Hert8]. Of notable concern, cardiovascular and metabolic diseases appear to greatly increase the risk of early death in those with SMI [Reference Correll, Solmi, Veronese, Bortolato, Rosson, Santonastaso, Thapa-Chhetri, Fornaro, Gallicchio, Collantoni, Pigato, Favaro, Monaco, Kohler, Vancampfort, Ward, Gaughran, Carvalho, Stubbs Correll, M and Solmi9], which is of particular importance, given the high prevalence of these diseases in SMI [Reference Correll, Solmi, Veronese, Bortolato, Rosson, Santonastaso, Thapa-Chhetri, Fornaro, Gallicchio, Collantoni, Pigato, Favaro, Monaco, Kohler, Vancampfort, Ward, Gaughran, Carvalho, Stubbs Correll, M and Solmi9–Reference Vancampfort, Stubbs, Mitchell, De Hert, Wampers, Ward, Rosenbaum, Correll, Vancampfort and Stubbs11]. People with SMI are also at increased risk of various other physical comorbidities, such as respiratory disease [Reference Partti, Vasankari, Kanervisto, Perälä, Saarni, Jousilahti, Lönnqvist, Suvisaari, Partti and Vasankari12, Reference Olfson, Gerhard, Huang, Crystal and Stroup13], poor bone health [Reference Stubbs, De Hert, Sepehry, Correll, Mitchell, Soundy, Detraux, Vancampfort, Stubbs and De Hert14] and physical multimorbidity [Reference Stubbs, Koyanagi, Veronese, Vancampfort, Solmi, Gaughran, Carvalho, Lally, Mitchell, Mugisha, Correll, Stubbs and Koyanagi15]. Moreover, people with SMI typically experience pronounced cognitive impairment, which often worsens over time [Reference Metzler, Dvorsky, Wyss, Müller, Gerstenberg, Traber-Walker, Walitza, Theodoridou, Rössler, Heekeren, Metzler and Dvorsky16–Reference Elias, Miskowiak, Vale, Köhler, Kjærstad, Stubbs, Kessing, Vieta, Maes, Goldstein, Carvalho, Elias and Miskowiak18] and for which treatment approaches remain limited [Reference Koster, Carbon and Correll19, Reference Carbon and Correll20].
Current treatment for mental health symptoms and functioning largely revolves around psychotropic medication [Reference Leucht, Cipriani, Spineli, Mavridis, Orey, Richter, Samara, Barbui, Engel, Geddes, Kissling, Stapf, Lässig, Salanti, Davis, Leucht and Cipriani21, Reference Correll, Rubio, Inczedy-Farkas, Birnbaum, Kane and Leucht22] and/or psychotherapeutic interventions [Reference Turner, van der Gaag, Karyotaki and Cuijpers23–Reference Cuijpers, Cristea, Karyotaki, Reijnders and Huibers25]. Whilst both of these dominant approaches, alone and in combination, have demonstrated treatment efficacy on mental health symptoms [Reference Huhn, Tardy, Spineli, Kissling, Förstl, Pitschel-Walz, Leucht, Samara, Dold, Davis, Leucht, Huhn and Tardy26], their impact on the rising physical health burden in this population is limited, and psychotropic medication may even have an adverse relationship with cardiometabolic/physical health [Reference Correll, Detraux, De Lepeleire and De Hert8, Reference Correll, Solmi, Veronese, Bortolato, Rosson, Santonastaso, Thapa-Chhetri, Fornaro, Gallicchio, Collantoni, Pigato, Favaro, Monaco, Kohler, Vancampfort, Ward, Gaughran, Carvalho, Stubbs Correll, M and Solmi9]. In addition, antipsychotic medication has been associated with reduced grey matter volume in people with schizophrenia [Reference Fusar-Poli, Smieskova, Kempton, Ho, Andreasen and Borgwardt27] while psychotherapeutic interventions appear to have limited efficacy for cognitive impairment in this population [Reference Cella, Preti, Edwards, Dow and Wykes28].
1.2. Established benefits of physical activity in the general population
In the general population, there is evidence that physical activity is equally effective as frontline pharmacological interventions, such as statins and beta-blockers, in preventing cardiovascular disease mortality [Reference Naci and Ioannidis29]. Moreover, there is consistent evidence that physical activity and exercise can decrease the risk of developing cardiovascular and metabolic disease [Reference Nelson, Rejeski, Blair, Duncan, Judge, King, Macera, Castaneda-Sceppa, Nelson and Rejeski30–Reference Haskell, Lee, Pate, Powell, Blair, Franklin, Macera, Heath, Thompson, Bauman, Haskell and Lee32] and reduce inflammatory parameters, such as C-reactive protein [Reference Hayashino, Jackson, Hirata, Fukumori, Nakamura, Fukuhara, Tsujii, Ishii, Hayashino and Jackson33, Reference Bergström, Behre and Schmidt34], which are commonly raised in people with SMI [Reference Köhler, Freitas, Maes, de Andrade, Liu, Fernandes, Stubbs, Solmi, Veronese, Herrmann, Raison, Miller, Lanctôt, Carvalho, Kohler and Freitas35]. Conversely, higher levels of sedentary behaviour (characterized by an energy expenditure ≤1.5 metabolic equivalents (METs), while in a sitting, reclining or lying posture during waking hours [36]) are independently associated with an increased risk of diabetes, cardiovascular disease and premature mortality [Reference Biswas, Oh, Faulkner, Bajaj, Silver, Mitchell, Alter, Biswas and Oh37]. In the general population, there is also evidence that lower levels of cardiorespiratory fitness are a more accurate determinant of premature death than body mass index (BMI) [Reference Kodama, Saito, Tanaka, Maki, Yachi, Asumi, Sugawara, Totsuka, Shimano, Ohashi, Yamada, Sone / Kodama and Saito38]. Moreover, there is evidence that aerobic exercise is effective in improving cognitive function in the general population [Reference Voelcker-Rehage and Niemann39–Reference Stanmore, Stubbs, Vancampfort, de Bruin and Firth43] including potentially increasing hippocampal volume [Reference Firth, Stubbs, Vancampfort, Schuch, Lagopoulos, Rosenbaum, Ward, Firth and Stubbs44]. In addition, a recent global meta-analysis has demonstrated that higher levels of PA confers protection from the development of depressive symptoms and MDD [Reference Schuch, Vancampfort, Firth., Rosenbaum., Ward, Silva, Hallgren, Dunn, Deslandes., Fleck, Carvalho and Stubbs45].
1.3. Low levels of physical activity and fitness
Despite the aforementioned, there is evidence to suggest that less than half of people with SMI (schizophrenia [Reference Stubbs, Firth, Berry, Schuch, Rosenbaum, Gaughran, Veronesse, Williams, Craig, Yung, Vancampfort, Stubbs and Firth46], bipolar disorder [Reference Vancampfort, Firth, Schuch, Rosenbaum, De Hert, Mugisha, Probst, Stubbs, Vancampfort and Firth47] and major depression [Reference Schuch, Vancampfort, Firth, Rosenbaum, Ward, Reichert, Bagatini, Bgeginski, Stubbs, Schuch and Vancampfort48] [Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha, Hallgren, Probst, Ward, Gaughran, De Hert, Carvalho, Stubbs, Vancampfort and Firth49]) meet recommended physical activity levels of 150 min of moderate-vigorous physical activity per week [50]. Moreover, each of these populations engage in remarkably high levels of sedentary behaviour [46 [Reference Schuch, Vancampfort, Firth, Rosenbaum, Ward, Reichert, Bagatini, Bgeginski, Stubbs, Schuch and Vancampfort48] and have low levels of cardiorespiratory fitness [Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst, Stubbs, Vancampfort and Rosenbaum51]. People with SMI experience, a number of barriers from engaging in physical activity exist, such as side effects of medications, complications from obesity/poor physical health [Reference Vancampfort, Correll, Probst, Sienaert, Wyckaert, De Herdt, Knapen, De Wachter, De Hert, Vancampfort and Correll52, Reference Vancampfort, Knapen, Probst, Scheewe, Remans and De Hert53], lack of resources/professional support [Reference Vancampfort, Stubbs, Sienaert, Wyckaert, De Hert, Rosenbaum, Probst, Vancampfort and Stubbs54], various motivational factors [Reference Firth, Rosenbaum, Stubbs, Gorczynski, Yung and Vancampfort55], which calls for targeted interventions in this highly sedentary population [Reference Vancampfort, Stubbs, Ward, Teasdale and Rosenbaum56–Reference Vancampfort, Rosenbaum, Probst, Connaughton, du Plessis, Yamamoto, Stubbs, Vancampfort and Rosenbaum58].
2. Aims
The overall aims of this meta-review and position statement were as follows: First, to establish the benefits of physical activity / exercise across all categories of severe mental illness (SMI), using top-tier evidence from published systematic reviews and meta-analyses. Second, examine how the benefits of physical activity may differ across specific SMIs, including schizophrenia-spectrum disorders, BD and MDD. Finally, to use these findings to provide guidance for clinical practice, policy and future research.
3. Methods
3.1. Guidance development process
This guidance paper was performed in accordance with the PRISMA guidelines [Reference Moher, Liberati, Tetzlaff and Altman59] following a pre-determined, published protocol (PROSPERO registration CRD42017068292). Moreover, the current guidance was conducted in accordance with the European Psychiatric Association (EPA) guidelines framework and wherever possible, adopted guidance based on the findings from systematic reviews and meta-analyses [Reference Heun and Gaebel60].
3.2. Searches and study selection
Two independent authors searched from inception to 15th January 2018 Medline/ Pubmed, PsychInfo, EMBASE and the Cochrane database for systematic reviews (with and without meta-analyses) of studies investigating physical activity/ exercise among people with SMI, schizophrenia-spectrum disorders, BD or MDD. The search terms included (exercise or aerobic exercise or physical activity or resistance training) and (schizophrenia or psychosis or psychotic or major depression or depression or bipolar disorder or serious mental illness or serious mental disorder). The reference lists of included articles were also hand-searched.
3.3. Type of studies eligible for inclusion
We followed the European Psychiatric Association manual for identifying and conducting this review and evidence was considered in a hierarchical approach, going from systematic reviews to the highest level of meta-analyses. In the absence of these two data sources for particular outcomes, we sought to identify newer randomised/controlled trials.
The specific inclusion criteria were: 1) systematic reviews (with or without meta-analyses) that synthesised randomised, controlled clinical trials or randomised control trials (RCTs) or controlled clinical trials (CCTs); 2) physical activity/ exercise interventions, including aerobic, high intensity and resistance exercise as monotherapy or in conjunction with other treatment options, including psychotropic medication or psychological therapies; 3) systematic reviews of PA, which included people with pooled SMI or schizophrenia-spectrum disorders, BD or MDD, confirmed through validated assessment measures (e.g. Diagnostic and Statistical Manual of Mental Disorders [61](DSM), International Classification of Diseases [Reference World Health organisation62] (ICD) criteria), in any setting; 4) systematic reviews, which included a non-active/ non-exercise control group (e.g., does not include physical activity). We excluded mind-body physical activity interventions, such as yoga and tai-chi, since these activities are presumed to exert beneficial effects on mental health through additional factors distinct from the physical activity itself.
3.4. Definition of exercise and physical activity
We included systematic reviews investigating the benefits of exercise or physical activity in people with SMI. Exercise was defined as “planned, structured, and repetitive and has as a final or an intermediate objective the improvement or maintenance of physical fitness.” [Reference Caspersen, Powell and Christenson63]. Within this definition, we included aerobic exercise, high intensity exercise, resistance exercise and mixed exercise (i.e., aerobic and resistance exercise). Physical activity was defined as “any bodily movement produced by skeletal muscles that results in energy expenditure” [Reference Caspersen, Powell and Christenson63]. We considered exercise/physical activity studies used as monotherapy or in combination with other types of treatment, e.g., psychotropic medication or psychological interventions.
3.5. Outcomes
As indicated in the EPA guidelines manual [Reference Heun and Gaebel60], as a first choice in the hierarchy of evidence, we drew evidence from systematic reviews of exercise/ physical activity interventions, including meta-analyses and/ or RCTs/ CCTs that considered the outcomes listed below. In accordance with our published protocol, we included data from the largest and/or most recent paper investigating each type of PA and each outcome in any population.
3.5.1. Primary outcomes
The primary outcomes focused on changes in the severity of symptoms, which characterise the included psychiatric disorders. For example, positive and negative symptoms in people with schizophrenia-spectrum disorders; and depressive symptoms in people with MDD or BD.
3.5.2. Secondary outcomes
We were interested in a range of secondary outcomes, including:
• Physical health factors, e.g., cardiovascular or metabolic parameter changes, anthropometric measures (e.g., BMI, waist circumference) or body composition measures (e.g., amount of intra-abdominal and cardiac adipose tissue).
• Cardiorespiratory fitness (expressed as percentage maximal or peak oxygen uptake), muscular fitness.
• Increasing physical activity levels or decreasing sedentary behaviour.
• Biomarkers, e.g., HbA1C, C-reactive protein, brain derived neurotrophic factor (BDNF), interleukin-6.
• Cognitive functioning, e.g. performance in neuropsychological tests
• Brain structure and connectivity, e.g., determined through magnetic resonance imaging or diffusor tension imaging changes.
• Quality of life and functioning.
• Dropout rates and predictors from physical activity interventions.
• Adverse events (e.g., injuries sustained from PA)
• Economic evaluations.
3.6. Data extraction
Data extraction was conducted by two authors and reviewed by a third author. We extracted data from systematic reviews and meta-analyses of RCTs/ CCTs investigating exercise interventions, including:
Number of studies included, number of participants in each arm, participant demographics, length of follow-up, details of the exercise intervention, statistical analyses conducted, effect size information, heterogeneity, publication bias and details of any meta regression and subgroup analyses.
Where a systematic review was not available for an outcome, we narratively summarized information from newer RCTs/ CCTs in the manuscript text, noting the study design, sample size, description of the intervention and control group, intervention effect and any harms and dropout rates.
3.7. Quality assessment and grading of evidence
Two independent authors assessed the quality of systematic reviews and meta-analyses with the AMSTAR tool [Reference Shea, Hamel, Wells, Bouter, Kristjansson, Grimshaw, Henry, Boers, Shea and Hamel64, Reference Shea, Grimshaw, Wells, Boers, Andersson, Hamel, Porter, Tugwell, Moher, Bouter, Shea and Grimshaw65]. In addition, we assessed the quality of evidence using the SIGN (2011) recommendations as indicated by the EPA guidelines [Reference Heun and Gaebel60] and in accordance with a recent published EPA guidelines on psychotherapy for depression [Reference Jobst, Brakemeier, Buchheim, Caspar, Cuijpers, Ebmeier, Falkai, Jan van der Gaag, Gaebel, Herpertz, Kurimay, Sabaß, Schnell, Schramm, Torrent, Wasserman, Wiersma, Padberg Jobst, EL and Brakemeier24]. Specifically, each included study was graded from 1++ (highest quality) to 4 (lowest quality) (see Table 1). Based on the quality of evidence, we graded recommendations according to best practice (Table 2).
4. Results
4.1. Search results and included studies
The initial database searches acquired 2722 hits and 2081 were reviewed at the abstract level. Subsequently, we reviewed 145 full texts, and 125 were excluded with reasons (see Fig. 1). Overall, 20 systematic reviews and meta-analyses were included in this guidelines document, which included studies that provided effect sizes for the benefit of exercise for SMI [Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst, Stubbs, Vancampfort and Rosenbaum51] (N = 1), schizophrenia-spectrum disorders [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67–Reference Vancampfort, Rosenbaum, Ward and Stubbs73] (N = 7), BD [Reference de Sá Filho, de Souza Moura, Lamego, Ferreira Rocha, Paes, Oliveira, Lattari, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Cheniaux, Yuan, Nardi, Machado de Sa Filho and de Souza Moura74, Reference Thomson, Turner, Lauder, Gigler, Berk, Singh, Pasco, Berk, Sylvia, Thomson and Turner75](N = 2) and MDD [Reference de Souza Moura, Lamego, Paes, Ferreira Rocha, Simoes-Silva, Rocha, de Sá Filho, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Yuan, Nardi, Machado, de Souza Moura and Lamego76–Reference Krogh, Hjorthoj, Speyer, Gluud and Nordentoft85] (N = 10).
Table 1 Grading of evidence in accordance with SIGN (2011 (and adapted from [Reference Jobst, Brakemeier, Buchheim, Caspar, Cuijpers, Ebmeier, Falkai, Jan van der Gaag, Gaebel, Herpertz, Kurimay, Sabaß, Schnell, Schramm, Torrent, Wasserman, Wiersma, Padberg Jobst, EL and Brakemeier24]).

Table 2 Grading of recommendations, modified from the SIGN (2011) and adopted from [Reference Schmidt, Schultze-Lutter, Schimmelmann, Maric, Salokangas, Riecher-Rössler, van der Gaag, Meneghelli, Nordentoft, Marshall, Morrison, Raballo, Klosterkötter and Ruhrmann Schmidt66].

Details of the included systematic reviews representing participants, interventions, outcomes, main results, considerations, AMSTAR scores and level of evidence scores are summarized in Table 3 for schizophrenia-spectrum disorders and mixed SMI participants, and in Table 4 for MDD and BD participants. Details of the results are described below for each population group.
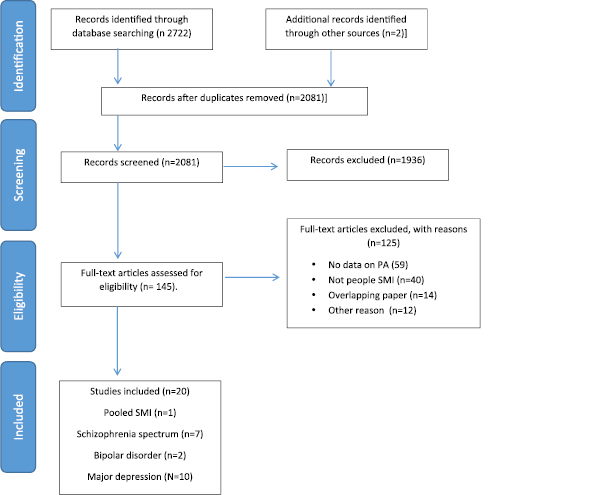
Fig 1. PRISMA flow-chart.
4.2. Pooled SMI
One meta-analysis provided non-overlapping data on exercise for people with pooled SMI [Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst, Stubbs, Vancampfort and Rosenbaum51, Reference Rosenbaum, Tiedemann, Sherrington, Curtis and Ward86]. It was demonstrated that aerobic exercise improved fitness in pre and post-test studies among people with schizophrenia-spectrum disorders and MDD (Hedges’ g = 0.33, 95% CI 0.21–0.45, p = 0.001, 13 studies) [Reference Vancampfort, Rosenbaum, Schuch, Ward, Richards, Mugisha, Probst, Stubbs, Vancampfort and Rosenbaum51] (AMSTAR 7, evidence level -1). Improvements in fitness were not accompanied by improvements in BMI. Meta regression showed that higher (vs, low or moderate) intensity interventions and those delivered by qualified professional predicted better outcomes. In 5 RCTs, exercise improved fitness versus control (Hedges’ g = 0.43, 95% CI 0.10–0.76, p = 0.01; n = 109).
4.3. Dropout, adverse events and economic evaluations of exercise interventions in pooled SMI
No information was reported on dropout rates, adverse events or economic evaluations in people with SMI.
4.4. Schizophrenia-spectrum disorders
In total, 7 systematic reviews and meta-analyses reported the benefits of exercise for schizophrenia-spectrum disorders [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67–Reference Vancampfort, Rosenbaum, Ward and Stubbs73]. There was broad variation in the frequency, intensity, type and time of the interventions across the included systematic reviews and meta-analyses (Table 3). The mean AMSTAR score for the included reviews was 6 (range 4–10).
A summary of the main results for exercise interventions in schizophrenia are presented in Table 5. In a meta-analysis of 8 RCTs [Reference Firth, Cotter, Elliott, French and Yung68] (AMSTAR 5, evidence level -1) and 659 patients with schizophrenia-spectrum disorders, exercise reduced total psychiatric symptoms when interventions used at least 90 min of moderate-to-vigorous physical activity (MVPA) per week (SMD: 0.72, 95% confidence interval −1.14 to −0.29). Ninety minutes of MVPA was also associated with reduced positive (SMDs -0.54, 95% CI -0.95 to -0.13) and negative symptoms (SMD-0.44, 95% CI -0.78 to -0.09). Pooled data from four RCTs found that exercise had no significant effect on reducing BMI (MD: -0.98 kg/m2; 95% CI -3.17 to 1.22 kg/m2, N = 4). Narrative findings regarding the influence of exercise on additional cardiometabolic parameters, such as waist circumference, high density lipoprotein levels and triglycerides, were inconsistent. Data from a narrative synthesis across 3 RCTs found that exercise also improved quality of life in people with schizophrenia-spectrum disorders. The key consideration from this systematic review was that optimal effects of exercise interventions are derived when people achieve over 90 min of MVPA per week.
A meta-analysis from 10 studies [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67] (AMSTAR 5, evidence level -1) including a sample of 186 in the intervention and 199 in the control group, found that exercise improved global cognition (g = 0.33, 95% CI = 0.13–0.53, P =.001). Meta-regression analyses indicated that greater improvements in global cognition were moderated by greater amounts of weekly exercise and when interventions were delivered by exercise professionals (e.g., physiotherapists, BSc level qualified exercise scientists). Sub-group analyses found that exercise improved cognitive domains of working memory (g = 0.39, P =.024, N = 7, n = 282), social cognition (g = 0.71, P =.002, N = 3, n = 81), and attention/vigilance (g = 0.66, P =.005, N = 3, n = 104). A systematic review of 14 trials [Reference Firth, Cotter, Carney and Yung69] investigated the pro-cognitive mechanisms of exercise in schizophrenia-spectrum disorders (AMSTAR 4, evidence level -1). The review suggested that exercise may increase brain volume, although there was a lack of consistency regarding which regions. One region that has been implicated is the hippocampus [Reference Pajonk, Wobrock, Gruber, Scherk, Berner, Kaizl, Kierer, Müller, Oest, Meyer, Backens, Schneider-Axmann, Thornton, Honer, Falkai, Pajonk and Wobrock87] although a meta-analysis of four studies did not support this hypothesis [Reference Firth, Stubbs, Vancampfort, Schuch, Lagopoulos, Rosenbaum, Ward, Firth and Stubbs44]. This review [Reference Firth, Cotter, Carney and Yung69] also found inconsistent evidence for an effect of exercise on BDNF levels, which is consistent with a meta-analysis [Reference Sanada, Zorrilla, Iwata, Bermúdez-Ampudia, Graff-Guerrero, Martínez-Cengotitabengoa, González-Pinto, Sanada, Zorrilla and wata72] (AMSTAR 10, evidence level -1) also finding that exercise did not influence BDNF levels. Thus, whilst there seems reasonable evidence that exercise does improve cognition in schizophrenia-spectrum disorders [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67], the underlying mechanisms are not yet clear.
A recent systematic narrative review [Reference Keller-Varady, Varady, Röh, Schmitt, Falkai, Hasan, Malchow, Keller-Varady and Varady88] investigated the influence of strength training on outcomes in schizophrenia-spectrum disorders (AMSTAR 4, evidence level -1). Across 6 studies (3 RCTs) the authors found tentative evidence that strength training over time can improve muscle strength, walking performance and reduce psychopathology symptoms. This systematic review was complimented by another systematic review [Reference Martin, Beard, Clissold, Androas and Currey71] (AMSTAR 7, evidence level -1) that found evidence that combined aerobic and strength training (7 RCTs) may improve strength, symptoms of schizophrenia and mental health, but not cardiovascular fitness.
A narrative systematic review [Reference Soundy, Muhamed, Stubbs, Probst and Vancampfort70] across 10 intervention studies (5 RCTs) (AMSTAR 5, evidence level -1) found that in those with schizophrenia-spectrum disorders, walking may reduce weight, BMI and body fat in the short-term. The authors noted that walking appeared to be safe, although the evidence was noted to be of medium-high risk of bias in the included studies.
Finally, a meta-analysis of seven studies (3 RCTs) [Reference Vancampfort, Rosenbaum, Ward and Stubbs73] (AMSTAR 8, evidence level -1) found that over a 12 week exercise intervention, aerobic exercise improved cardiorespiratory fitness versus control conditions (g = 0.41, 95% = CI 0.05 to 0.82, P = 0.028).
4.5. Dropout, adverse events and economic evaluations of exercise interventions in schizophrenia-spectrum disorders
A meta-analysis of 19 RCTs and 594 schizophrenia-spectrum disorder patients [Reference Vancampfort, Rosenbaum, Schuch, Ward, Probst and Stubbs89] demonstrated that 26.7% [95% confidence interval (CI) = 19.7%–35.0%] of patients dropped out of interventions, more than double reported in non-active control interventions (odds ratio = 2.15, 95% CI = 1.29–3.58, P =.003). Factors influencing lower dropout in meta-regression analyses included supervision from a recognized exercise professional and including a motivational component.
Surprisingly, very little information is available on the potential harms or adverse events from exercise in schizophrenia-spectrum disorder patients. Specifically, the following papers did not report harms [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67–Reference Firth, Cotter, Carney and Yung69, Reference Martin, Beard, Clissold, Androas and Currey71, Reference Sanada, Zorrilla, Iwata, Bermúdez-Ampudia, Graff-Guerrero, Martínez-Cengotitabengoa, González-Pinto, Sanada, Zorrilla and wata72, Reference Keller-Varady, Varady, Röh, Schmitt, Falkai, Hasan, Malchow, Keller-Varady and Varady88]. Only one study [Reference Soundy, Muhamed, Stubbs, Probst and Vancampfort70] reported potential harms and found that walking was not associated with adverse events in schizophrenia-spectrum disorders.
To the best of our knowledge, there have been no studies with an economic evaluation of the cost and value of delivering exercise interventions in schizophrenia-spectrum disorders.
4.6. Major depression
In total 10 systematic reviews and meta-analyses reported the benefits of exercise for MDD [Reference de Souza Moura, Lamego, Paes, Ferreira Rocha, Simoes-Silva, Rocha, de Sá Filho, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Yuan, Nardi, Machado, de Souza Moura and Lamego76–Reference Krogh, Hjorthoj, Speyer, Gluud and Nordentoft85]. The mean AMSTAR score for the included reviews was 9.1 (range 3–10). Full details of the included systematic reviews are summarized in Table 4.
A summary of the effects of exercise in MDD are reported in Table 5. In a comprehensive Cochrane review [Reference Cooney, Dwan, Greig, Lawlor, Rimer, Waugh, McMurdo, Mead, Cooney and Dwan83] including exercise trials, the authors found that exercise was effective in reducing depressive symptoms versus control conditions (Hedge’s g=-0.68, 95% CI -0.92 to -0.44, p = 0.001, I² = 67.99, k = 23) (AMSTAR 11, evidence level 1+). Exercise was effective in studies with (Hedge’s g = -0.40, 95% CI = -0.70 to -0.11, P = 0.01, k = 10) or without (Hedge’s g=-0.91, 95% CI = -1.22 to -0.61, P < 0.001, k = 13) blinded outcome, with (Hedge’s g=-0.56, 95% CI -0.87 to -0.25, p < 0.001, k = 12) or without (Hedge’s g =-0.80, 95% CI = -1.15 to -0.46, p < 0.001, k = 11) intention-to-treat, and in studies comparing exercise with no intervention (Hedge’s g = -1.24, 95% CI = -1.83 to -0.65, k = 4), and with usual care (Hedge’s g = -0.48, 95% CI = -0.8 to -0.16, k = 4). There was no difference in exercise versus psychological interventions (Hedge’s g = -0.22, 95% CI -0.65 to 0.21, k = 4), or antidepressant treatment (Hedge’s g = -0.08, 95% CI = -0.33 to 0.18, k = 3), indicating exercise demonstrated comparable efficacy versus these established interventions. However, information on symptom severity was not available. Of note, beneficial effects were confirmed when considering only studies with blinded outcomes (Hedge’s g = -0.40, 95% CI -0.69 to -0.11, P = 0.01, k = 10), but not when considering blinded outcomes with both adequate allocation concealment and intention-to-treat together (Hedge’s g = -0.26, 95% CI = -0.61 to 0.08, P = 0.14, k = 6). Also, at longer follow-up (mean 10.75 month) no significant effect was reported (Hedge’s g =-0.22, 95% CI -0.53 to 0.09, P = 0.16, k = 7).
Table 3 Characteristics and results of systematic reviews and meta-analyses including studies comparing effects of PA with a control group in Schizophrenia and mixed SMI participants.
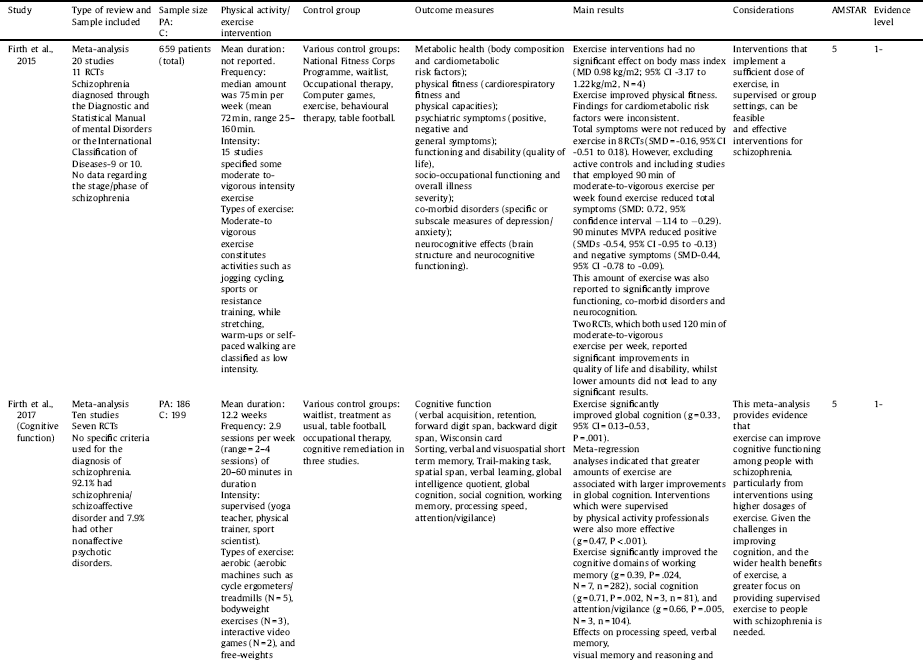



Key: BDNF = Brain-derived neurotrophic factor, C = control, CI = confidence intervals, DSM = Diagnostic and Statistical Manual of mental Disorders, PA = physical activity, RCT = randomized controlled trial, SMD = standardized mean difference.
Table 4 Characteristics and results of systematic reviews and meta-analyses including studies comparing effects of PA with a control group in major depression and bipolar disorder.
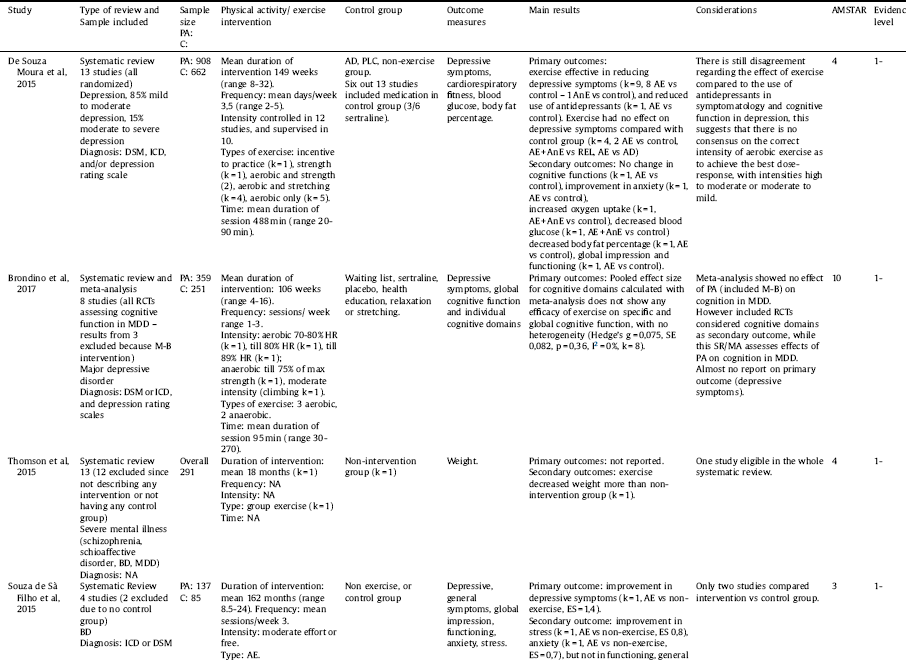




Key: AD = antidepressant treatment, AE = aerobic exercise, AnE = anaerobic exercise, BMI = body mass index, BP = blood pressure, C = control, CL = climbing, CRF: cardiorespiratory fitness; CRP: C-reactive protein, EDU = educational intervention, ES = effect size, MB = mind-body, MDD = major depressive disorder; NA = not available, PA = physical activity, PLC = placebo, RCT = randomized controlled trial, REL = relaxation, ST = stretching, WT = waiting list.
Table 5 Summary of included systematic review results and quality of the evidence.
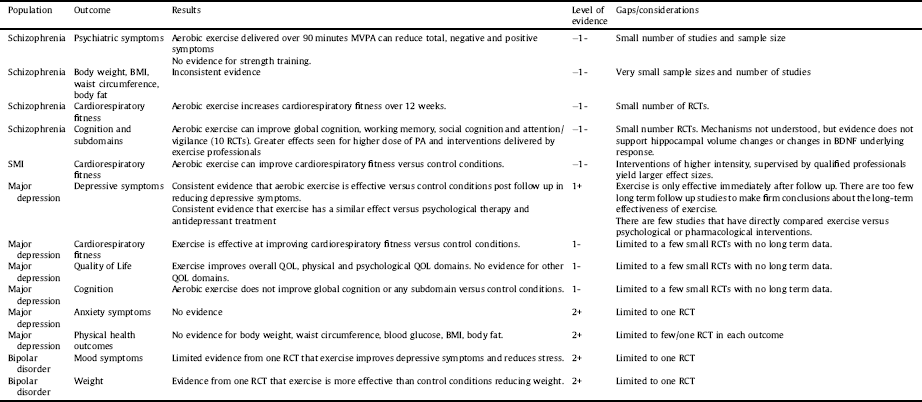
Key: MVPA = moderate-vigorous physical activity, BMI = body mass index, PA = physical activity.
More recently, a meta-analysis [Reference Schuch, Vancampfort, Richards, Rosenbaum, Ward and Stubbs81] (AMSTAR 10, evidence level 1+) attempted to address some of the criticisms of the Cochrane review [Reference Cooney, Dwan, Greig, Lawlor, Rimer, Waugh, McMurdo, Mead, Cooney and Dwan83], such as the inclusion of a number of in-eligible trials, comparing exercise versus other exercise interventions, and the absence of adjusting for publication bias. The authors [Reference Schuch, Vancampfort, Richards, Rosenbaum, Ward and Stubbs81] found across 25 RCTs (intervention 757, control 730) that exercise versus non-active interventions over a mean of 14 weeks resulted in a large improvements of depressive symptoms (SMD = 1.11, 95% CI% = 0.79–1.43, k = 25) with a failsafe number of 1057. Exercise was effective both when the diagnosis was made according to DSM [61] (SMD = 1.14, 95% CI = 0.46–1.81, P < 0.001, I² = 88.5, k = 9) or depressive symptoms (SMD = 0.801, 95% CI = 0.49–1.11, P < 0.001, I² = 68.5, k = 14), high (SMD = 0.882, 95% CI = 0.221–1.544, P = 0.009, I² = 90.1, k = 4) and low (SMD = 1.033, 95% CI = 0.66–1.41, P < 0.001, I² = 79.2, k = 21) quality studies, outpatients (SMD = 1.123, 95% CI = 0.77–1.47, p < 0.001, I2 = 84.6, k = 21) and inpatients (SMD = 0.553, 95% CI = 0.167-0.938, P = 0.005, I² = 0, k = 3), but not in a nursing home (k = 1). Exercise was effective at moderate (SMD = 1.33, 95% CI 0.46–2.197, P = 0.003, I² = 83.4, k = 6) and vigorous (SMD = 1.342, 95% CI = 0.437–2.246, P = 0.004, I² = 91.1, k = 7), but not at light intensity (k = 3), and was effective as aerobic exercise (SMD = 1.045, 95% CI = 0.653–1.437, P < 0.001, I² = 80.97). The same author group also reported that exercise was effective in improving depressive symptoms in older adults (SMD = -0.90, 95%CI = 0.28–1.51, P = 0.004, I [Reference Walker, McGee and Druss2] = 81.63, P = 0.001, k = 8). In addition, a recent meta-analysis [Reference Kvam, Kleppe, Nordhus and Hovland82] (AMSTAR 10, evidence level 1+) confirmed that exercise was effective in reducing depressive symptoms, including those with and without blinded outcomes and with or without intention-to-treat analysis (see Table 4). The authors [Reference Kvam, Kleppe, Nordhus and Hovland82] also confirmed results from the Cochrane review that exercise was as effective as psychological intervention and antidepressant treatment for depressive symptoms (see Table 4). In another review regarding mental health symptoms [Reference Silveira, Moraes, Oliveira, Coutinho, Laks and Deslandes84] (AMSTAR 7, evidence level -1), exercise increase response but not remission of MDD (see Table 4). Finally, a trial sequential meta-analysis [Reference Krogh, Hjorthoj, Speyer, Gluud and Nordentoft85] (AMSTAR 10, evidence level -1) confirmed earlier results that exercise was effective for MDD (SMD − 0.66, 95% CI − 0.86 to −0.46; p < 0.001). Moreover, the authors tentatively suggested that when pooling four RCTs of “high quality” with a trial sequential meta-analysis (two of which were their own studies and concerns have been raised about the inappropriate use of active control groups [Reference Ekkekakis90]) that exercise may no longer be effective.
A narrative systematic review [Reference de Souza Moura, Lamego, Paes, Ferreira Rocha, Simoes-Silva, Rocha, de Sá Filho, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Yuan, Nardi, Machado, de Souza Moura and Lamego76] (AMSTAR 4, evidence level -1) suggested that exercise did not influence anxiety symptoms, oxygen uptake, blood glucose, body fat or functioning versus control conditions, although all of these outcomes were limited to one study only. A recent meta-analysis of 8 RCTs [Reference Brondino, Rocchetti, Fusar-Poli, Codrons, Correale, Vandoni, Barbui, Politi and Brondino Rocchetti77] (AMSTAR 10, evidence level -1) found that aerobic exercise was not effective for global cognition (Hedges' g = 0.07, 95% CI = -0.08 to 0.24, I² = 0%) nor for any cognitive subdomains. A meta-analysis of 7 RCTs [Reference Stubbs, Rosenbaum, Vancampfort, Ward and Schuch78] demonstrated that aerobic exercise over approximately 12 weeks improved cardiorespiratory fitness (Hedge’s g = 0.64, 95%CI = 0.32–0.96, P = 0.001, I2 = 67%, k = 8) (AMSTAR 10, evidence level 1+). Exercise also improved quality of life (QOL) overall (SMD = 0.39, 95%CI = 0.47–0.74, P = 0.002, I² = 0.0, k = 5) in addition to physical (SMD = 0.53, 95%CI = 0.22–0.84, P = 0.001, I² = 00.00, k = 5) and psychological (SMD = 0.53, 95%CI = 0.22–0.85, P = 0.001, I² = 4.89, k = 5) QoL domains. However, no effect was seen in social (SMD = 0.28, 95%CI = -0.13 to 0.71, p = 0.18, I² = 13.04, k = 5) or environmental QoL domains (SMD = 0.36, 95%CI = -0.12 to 0.85, P = 0.14, I² = 30.75, k = 5).
4.7. Dropout, adverse events and economic evaluations of exercise interventions in major depression
A meta-analysis of 31 RCTs [Reference Stubbs, Vancampfort, Rosenbaum, Ward, Richards, Soundy, Veronese, Solmi, Schuch, Stubbs and Vancampfort91] reported that the dropout rate adjusted for publication bias was 17.2% (95%CI = 13.5–21.7) in MDD only. In MDD participants, higher baseline depressive symptoms (β = 0.0409, 95%CI = 0.0809-0.0009, P = 0.04) predicted greater dropout, whilst supervised interventions delivered by physiotherapists (β = -1.2029, 95%CI = -2.0967 to -0.3091, P = 0.008) and exercise physiologists (β = -1.3396, 95%CI = -2.4478 to -0.2313, P = 0.01) were associated with lower dropout. A comparative meta-analysis (N = 29) established dropout was lower in exercise than control conditions (OR = 0.642, 95%CI = 0.43-0.95, P = 0.02).
Information on adverse events was limited in the reported systematic reviews. One review [Reference Cooney, Dwan, Greig, Lawlor, Rimer, Waugh, McMurdo, Mead, Cooney and Dwan83] reported that 6 RCTs reported adverse events. These included muscular pain/symptoms, fatigue and increased depressive symptoms, but there was no difference versus those reported in control conditions. Another review confirmed there were no differences in serious or non-serious adverse events versus control conditions [Reference Krogh, Hjorthoj, Speyer, Gluud and Nordentoft85].
To the best of our knowledge, there have been no economic evaluations of the cost effectiveness of delivering exercise interventions for people with MDD.
4.8. Bipolar disorder
In total, 2 systematic reviews reported the benefits of exercise for BD [Reference de Sá Filho, de Souza Moura, Lamego, Ferreira Rocha, Paes, Oliveira, Lattari, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Cheniaux, Yuan, Nardi, Machado de Sa Filho and de Souza Moura74, Reference Thomson, Turner, Lauder, Gigler, Berk, Singh, Pasco, Berk, Sylvia, Thomson and Turner75]. Both systematic reviews were rated with an AMSTAR score of 4. The evidence was very limited because of the number of eligible studies in BD patients. A summary of the effects of exercise in BD are reported in Table 5. One review identified only two studies that included people with BD, [Reference de Sá Filho, de Souza Moura, Lamego, Ferreira Rocha, Paes, Oliveira, Lattari, Rimes, Manochio, Budde, Wegner, Mura, Arias-Carrión, Cheniaux, Yuan, Nardi, Machado de Sa Filho and de Souza Moura74] finding some evidence that exercise may improve depressive symptoms and reduce stress, but observing no influence on function, general symptoms or clinical global impression. In the other systematic review, only one study was identified in BD patients, 75] which suggested that exercise decreased body weight more than in the control group.
4.9. Dropout, adverse events and economic evaluations of exercise interventions in bipolar disorder
We found no reports in the included systematic reviews of dropout rates, adverse events or economic evaluations.
4.10. Recommendations
Based on our extensive systematic review of the literature, we have made ten recommendations for future research (where gaps are evident) and clinical practice based on the degree of evidence and grades in line with the SIGN methodology checklist. In line with the SIGN and previous EPA guidelines, [Reference Schmidt, Schultze-Lutter, Schimmelmann, Maric, Salokangas, Riecher-Rössler, van der Gaag, Meneghelli, Nordentoft, Marshall, Morrison, Raballo, Klosterkötter and Ruhrmann Schmidt66] recommendations were graded as A (meta-analysis, systematic review or other level 1 evidence), B (meta-analysis, systematic review or RCT with moderate or high risk of bias), C (large body of evidence from intervention studies, observational data), or D (expert opinion).
5. Research recommendations
5.1. For all SMI groups, more research is needed into the effects of PA interventions during the prodromal and early phase of illness onset (Based on expert opinion, D)
The majority of research on the effect of PA has been conducted in patients with established SMI. However, in the case of physical health outcomes, there is evidence from the general population that using lifestyle changes to prevent deterioration in metabolic health is a more feasible and effective approach than attempting to reverse chronic cardio-metabolic diseases [Reference Schellenberg, Dryden, Vandermeer, Ha and Korownyk92]. Research is needed to determine whether exercise interventions in the early stages of SMI likewise have a positive effect on physical and mental health outcomes. Furthermore, research is needed to assess whether using exercise to improve mental health and psychosocial functioning shortly after illness onset may reduce the likelihood of long-term functional disability, or increase the likelihood of achieving a full and sustained recovery. Given this situation, we recommend a greater research emphasis on evaluating PA interventions during the prodromal phase and first/early presentation of SMI.
5.2. To optimize treatment ‘reach’, research should focus on establishing pragmatic, scalable methods for delivering PA as a treatment for SMI (Based on expert opinion, D)
Research priorities should shift from efficacy studies to pragmatic effectiveness trials. Specifically, there is a need to develop replicable and scalable methods for delivering PA interventions to people with SMI, in a format which is accessible, engaging and effective for large numbers of patients. There is sufficient evidence to indicate that the most effective and engaging interventions will be those that are (i) delivered by qualified exercise professionals (rather than mental health staff), and (ii) delivered at sufficient levels of intensity to significantly improve physical capacities, such as cardiorespiratory fitness. Whilst the evidence is clear that better treatment outcomes and less dropout are achieved across SMI for PA interventions delivered by qualified exercise professionals, the potential benefits of training mental health staff (e.g., psychologists, psychiatric nurses - of whom there are many more) on principles of PA in delivering exercise interventions should be evaluated.
5.3. Controlled trials of exercise for SMI should aim to determine the optimal dose-response of PA needed to treat SMI and should focus on implementation and culture in clinical practice (Based on expert opinion, D)
Whilst there is some evidence from exercise trials in MDD that moderate/high intensity PA is associated with larger effect sizes than low-intensity PA for mental health and cardiorespiratory fitness [Reference Schuch, Vancampfort, Richards, Rosenbaum, Ward and Stubbs81] and for better cognitive outcomes in established schizophrenia [Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch, Elliott, Nuechterlein, Yung Firth and Stubbs67], more research is specifically required to ascertain the optimum frequency, intensity, time and type of interventions in each SMI, although it is important to note this may vary for each individual based on their preferences and/or illness characteristics. In addition, very few systematic reviews reported adverse events. Future studies should carefully assess any adverse events, particularly in light of any cardiovascular risk and events. This may include screening for cardiovascular risk and appropriately individualizing the optimal frequency and intensity of PA, which has been covered extensively elsewhere [Reference Vancampfort, Rosenbaum, Probst, Soundy, Mitchell, De Hert and Stubbs Vancampfort Rosenbaum93, Reference De Hert, Dekker, Wood, Kahl, Holt and Möller94]. Of most importance, future translational research focussing on implementation into clinical practice is urgently required. This should involve determining optimal motivational frameworks and understanding cultures in practice that enable the optimal uptake and maintenance of PA.
5.4. Research examining the effects of interventions to reduce sedentary behaviours and increase PA is needed (Based on expert opinion, D)
Recent meta-analyses have demonstrated that people with SMI are highly sedentary [Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha, Hallgren, Probst, Ward, Gaughran, De Hert, Carvalho, Stubbs, Vancampfort and Firth49]. With increasing evidence that sedentary behaviours have independent adverse effects on somatic health [Reference Biswas, Oh, Faulkner, Bajaj, Silver, Mitchell, Alter, Biswas and Oh37], research is needed to investigate if sedentary behaviours can be reduced in this population and tease out the relative importance of reducing sedentary behaviours in the context of structured exercise interventions.
5.5. The underlying neurobiological mechanisms of PA in SMI are inadequately understood and require further investigation (Based on expert opinion, D)
Several tenable theories have been proposed to explain the positive effects of PA in SMI, but the results are unclear. In particular, it is uncertain what ‘dose’ of exercise is needed to elicit the purported biological mechanisms, and how these interact with the psycho-social benefits of exercise. Future research should explore the underlying mechanisms of PA efficacy in all SMIs.
5.6. Undertake long term and cost-effectiveness analyses of PA interventions (Based on expert opinion, D)
Whilst there is an increasing evidence base on the efficacy of PA for SMI, there is an absence of cost effectiveness studies. Undertaking cost-effectiveness research should be a priority. There are also relatively few long term (≥12 months) trials, although a recent large RCT lasting 12 months demonstrated that PA was as effective as internet-delivered CBT, and both were better than the control group [Reference Hallgren, Kraepelien, Öjehagen, Lindefors, Zeebari, Kaldo, Forsell, Hallgren and Kraepelien95].
6. Clinical practice recommendations
6.1. Physical activity should be used as a treatment for mild-moderate depression to improve symptoms and physical fitness (good evidence, A)
There is now an abundance of data demonstrating that as an adjunctive treatment, PA is effective in reducing depressive symptoms in people with MDD. Specifically, based on current evidence, we recommend that intervention consists of 2–3 sessions of supervised aerobic and/or aerobic and resistance training exercise a week of 45–60 minutes duration of moderate intensity. The literature regarding resistance training as a standalone intervention for MDD is limited, but growing. To date, there have been limited direct head-to-head comparisons of different types of exercise interventions (in terms of frequency, intensity, time and type), which precludes definitive conclusions being made about the superiority or better acceptability (or lack thereof) of specific forms of PA, and our current knowledge is derived primarily from interventions comparing “PA” versus non-active interventions. In order to achieve optimal outcomes and less dropouts, the evidence base indicates that physiotherapists/qualified exercise professionals should lead and supervise PA interventions. However, since qualified exercise professionals are not available in each setting, the efficacy and effectiveness of training other personnel in the principles and delivery of PA requires further study. Finally, there is also good evidence that PA can improve cardiorespiratory fitness in people with depression [Reference Stubbs, Rosenbaum, Vancampfort, Ward and Schuch78].
6.2. Physical activity should be utilised as an adjunctive treatment for schizophrenia-spectrum disorders, to improve symptoms, cognition and QoL (good evidence, B)
Our review indicates that PA is an effective adjunct to improve symptoms, global cognition and various subdomains, and quality of life in schizophrenia-spectrum disorders. In fact, at least in indirect comparison, the benefits of PA for cognition seem to be comparable to other psychological interventions (e.g., cognitive remediation) and should be a core part of multidisciplinary treatment. The most consistent evidence to date is available for aerobic exercise that meets 150 min of moderate to vigorous PA per week. There are limited data available specifically on resistance training for people with schizophrenia-spectrum disorders and data are inadequate comparing the potential superiority of various forms of PA to each other. Nonetheless, interventions supervised by qualified professionals, with a motivational component result in less dropout.
6.3. Physical activity should be used to improve physical health in people with SMI (Some evidence, C)
Whilst there is an abundance of evidence for PA to prevent the onset of a number of physical diseases (e.g., cardiovascular disease) and to have the potential to improve these conditions in the general population, the evidence base in individual SMIs is mixed, possibly due to poor adherence and the paucity of studies that have targeted this important topic. However, there is some indication that PA can improve cardiovascular disease risk markers in people with SMI. Given the weight of evidence in the general population and some initial data in people with SMI, we advocate that PA forms a core part of preventing and managing poor physical health in people with SMI. Nevertheless, more research should be performed in this area to further strengthen the support for this recommendation.
6.4. People with SMI should be screened for PA habits in primary and secondary care (Based on expert opinion, D)
In light of the evidence of the health benefits of PA, the use of brief PA screening in primary/secondary care in the general population as a health indicator and to manage chronic conditions is common [Reference Golightly, Allen, Ambrose, Stiller, Evenson, Voisin, Hootman, Callahan, Golightly, KD and Allen96, Reference Carlson, Maynard, Fulton, Hootman and Yoon97]. A previous systematic review [Reference Soundy, Roskell, Stubbs and Vancampfort98] identified, no specific self-report PA tool has robust psychometric properties and can be advocated for routine use in clinical practice. Nonetheless, given that people with all SMIs are known to engage in low levels of PA and high SB [Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha, Hallgren, Probst, Ward, Gaughran, De Hert, Carvalho, Stubbs, Vancampfort and Firth49] a routine measure of PA to monitor PA habits could prove useful. To the best of our knowledge, only one PA tool, the SIMPAQ [Reference Rosenbaum and Ward99] has been developed specifically for use to measure PA and SB in clinical practice. Given the above literature, we advocate that people with SMI are screened for PA and advised on increasing activity levels in primary and secondary services. Further research is needed to identify a practical and valid measure that can be used in clinical care.
7. Conclusion
There is now considerable empirical evidence supporting the use of PA interventions in the treatment of MDD and schizophrenia-spectrum disorders. Specifically, our meta-review supports the use of aerobic exercise of moderate-vigorous intensity at a frequency of 2–3 times a week, ideally supervised by qualified professionals and achieving 150 min of MVPA per week in order to improve outcomes in people with MDD and schizophrenia-spectrum disorders. There is also some evidence that a combination of aerobic and resistance training meeting the above frequency, intensity and time criteria can improve outcomes in people with MDD and schizophrenia-spectrum disorders. There is growing interest in the use of resistance training as a standalone PA intervention. However, the literature is equivocal in both MDD and schizophrenia-spectrum disorders. Currently, the evidence for use of PA in patients with BD is promising, but limited by the paucity of studies, and it is therefore not possible to reach any firm conclusions regarding population-specific recommendations above and beyond those aimed at the general population. Across all disorders, there have been limited interventions that have specifically compared various forms of PA versus one another, which again precludes reaching any firm conclusions based on the relative superiority of the different forms of PA, and one should be aware that our knowledge to date is largely drawn from studies comparing PA versus non-active control conditions. The underlying psychobiological mechanisms of the benefits derived from PA are only poorly understood, and further research is needed to elucidate causal relationships between exercise and improvements in the mental and physical domains of people with SMI. Finally, due to absence of data, it is currently unclear whether PA is a cost-effective treatment option, and more work is needed to establish the financial aspects of this mode of treatment compared with conventional approaches, taking into account the various domains of lives of people with SMI that PA can affect.
Funding
This research received no specific funding. However, BS and FG are supported in part by the Maudsley Charity and National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust. BS is supported by the Health Education England and the National Institute for Health Research HEE/ NIHR ICA Programme Clinical Lectureship (ICA-CL-2017-03-001). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care
Conflict of interest
BS, DV, JF, MH, MS, MG, NV, FK have no conflicts of interest to declare.
CUC has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Angelini, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Merck, Neurocrine, Otsuka, Pfizer, ROVI, Servier, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, ROVI and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a shareholder of LB Pharma. FG has received honoraria for advisory work and lectures from Roche, BMS, Lundbeck, and Sunovion and has a family member with professional links to Lilly and GSK.









Comments
No Comments have been published for this article.