No CrossRef data available.
Article contents
Dramatic Improvement in Outpatient Opiate-free Exits Using a Novel Resource-efficient Lofexidine-based Protocol (bristol-regime) – a Service Evaluation Study
Published online by Cambridge University Press: 15 April 2020
Abstract
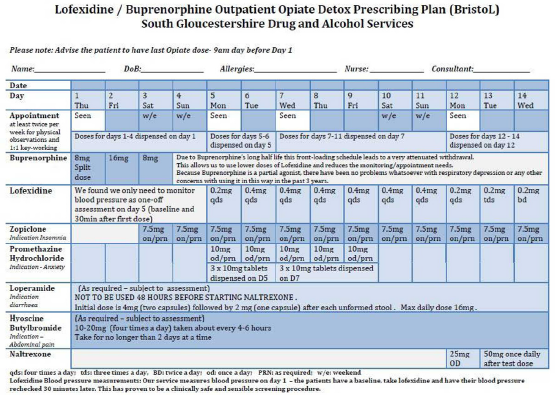
Lofexidine is an alpha-2-A noradrenergic receptor agonist approved in the United Kingdom for treating opioid withdrawal symptoms. Due to relatively poor detox success rates locally, we introduced a novel regime – the BristoL (Buprenorphine-Lofexidine) protocol (table 1):
Only four outpatient appointments over 14 days.
Buprenorphine front-loading (days 1-3)
Lofexidine (days 5-14)
Naltrexone offered on day 12
We assessed the efficacy of this regime in our outpatients (annual numbers coming into treatment 120-150/year) compared to previous client-led regimes.
Retrospective case notes review and electronic patient management software were used to calculate changes in opiate-free exits.
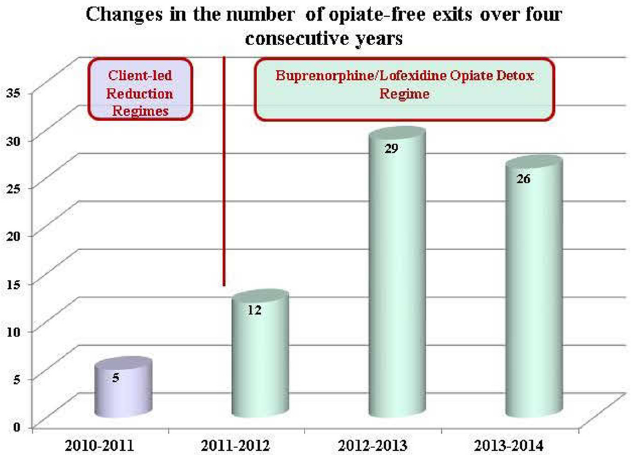
Drug free exits in the first year after introducing the BristoL protocol dramatically increased by 140% (from 5 to 12) and doubled again the following year (29) with comparable figures the year after (26) demonstrating a sustained effect (figure 1).
Introducing the BristoL protocol led to a dramatic improvement in opiate-free exits over three years with good tolerability and no significant side effects. Its advantages – simplified prescribing, reduced monitoring and a dramatically improved reported patient experience – have meant we are now also using it in the primary care setting.
- Type
- Article: 0475
- Information
- European Psychiatry , Volume 30 , Issue S1: Abstracts of the 23rd European Congress of Psychiatry , March 2015 , pp. 1 - 2
- Copyright
- Copyright © European Psychiatric Association 2015



Comments
No Comments have been published for this article.