1. Introduction
Approximately 100 years ago, Bleuler famously declared that “Sensory response to external stimulus is quite normal” in schizophrenia [Reference Bleuler1], followed however by the cryptic statement: “Busch and Kraepelin have found in perception experiment (using the shutter and revolving drum apparatus) that schizophrenics show many more errors and particularly omissions than do the healthy…Using accurate apparatus, we were unable to substantiate these findings” [Reference Bleuler1].
Bleuler’s statements led to a century of formulations of schizophrenia in which perceptual deficits were de-emphasized relative to other types of cognitive operations, including the well-known four” A”s of Bleuler (i.e., [disturbance of] affect, association, ambivalence and autism). This viewpoint addresses the concept of sensory/perceptual dysfunction in schizophrenia, and reconstructs the Busch/Kraepelin experiment alluded to by Bleuler. We argue that with the benefit of hindsight, the findings of Busch/Kraepelin were probably not only correct but also prescient, and that sensory deficits remain a critical, albeit underemphasized, feature of schizophrenia. As in the time of Kraepelin and Bleuler, technical issues as well as theoretical formulations may still be contributing to the de-emphasis of sensory/perceptual deficits vs. other aspects of cognitive impairment in schizophrenia.
2. Early history of sensory studies in schizophrenia
2.1. Visual sensory deficits
When Kraepelin first described the syndrome of Dementia Praecox in the late 1800s, he believed that genetically based generalized processes, such as autointoxication, led to the clinical manifestations. Subsequently, Kraepelin characterized Dementia Praecox as a whole-brain neurodegenerative disorder similar to senile dementia (Alzheimer’s disease), but affecting younger individuals. Consequently, extensive research was devoted to characterizing patients “neurological” as well as psychological functions [Reference Engstrom and Weber2].
One type of test device used at that time was the “Cron-Kraepelin” apparatus, which presented letters written on a rotating drum through a 2-mm aperture (Fig. 1A). With this apparatus, the length of stimulus presentation could be controlled by altering the rotation speed of the drum. With this device, Kraepelin and colleagues were able to demonstrate significant deficits in detection of briefly presented stimuli. However, the need for the drum to complete full slow rotations limited the utility of the device.
A technological advance occurred with the development of an advanced electromechanical apparatus - the “schußplatte” (Fig. 1B) - that allowed presentation of stimuli for as short as 1-ms, and was easier to apply than the rotating drum. As with the rotating drum experiments, subjects were asked to identify briefly presented letters. The experiment by Busch using this apparatus was published in 1908 [Reference Busch3] in Kraepelin’s journal (reproduced in Supplemental Material), and contains sufficient subject-level data to permit modern re-analysis.
The study included 17 patients diagnosed as dementia praecox, and 6 healthy individuals. Stimuli were presented with a duration of 16.7 ms. Percent correct detections were measured at 0, 10 and 30 s following presentation and summarized per subject. Although statistical analysis was not conduced at the time, in modern terms, the effect-sizes between groups were extremely large (d>1.0) and highly significant even in the 0 s delay condition (Fig. 1C).
Although Bleuler stated that his group was unable to substantiate Kraepelin and colleagues’ findings, no experimental methods or data were presented. Thus, the quality of the replication study cannot be assessed. Deficits in early visual processing were not rediscovered until the studies of Holzman and colleagues starting in the early 1970’s that showed impaired smooth pursuit during eye-tracking, followed by the studies of Braff and colleagues demonstrating increased critical stimulus duration and “transient” visual system dysfunction in schizophrenia (rev. in Ref. [Reference Javitt4]).
In modern studies, as in the initial Busch/Kraepelin experiment, schizophrenia patients show reliable, highly significant increases in time required for visual object recognition (“critical stimulus duration”) especially to stimuli biased towards the magnocellular visual pathway [Reference Schechter, Butler, Silipo, Zemon and Javitt5]. This pathway provides critical input into the dorsal stream (often referred as the “where” pathway or “vision for action” system), which is particularly important for the rapid detection of low contrast, low spatial frequency and motion features of stimuli, for processing of information within peripheral visual fields, and for rapidly guiding eye movements towards salient features of the environment [Reference Javitt4].
Unlike the alternative “vision for identification” system, individuals are not consciously aware of the functioning of their magnocellular system. For example, in response to peripheral motion, eye movements may be initiated even before a person is consciously aware of having seen the stimulus. Thus, while some patients spontaneously report visual changes during the early stages of their illness [Reference Javitt4], most subjects with magnocellular dysfunction are simply unaware of their visual deficit.
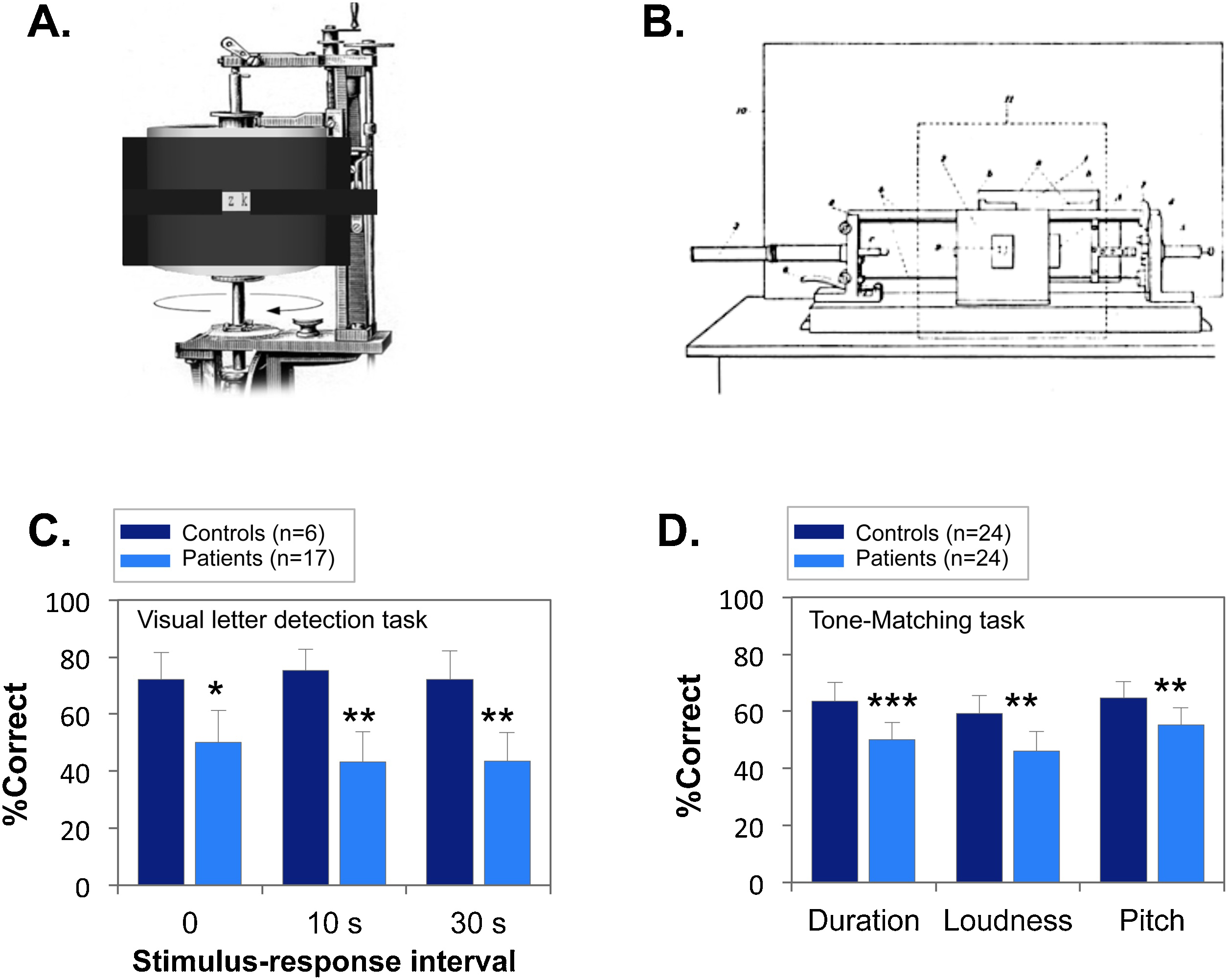
Fig. 1. A. Rotating-drum. (image from: Zimmermann. 1928, S. 185: nachbearbeitet von © 2009 H. Maxiilian Wontora).
The apparatus shows 3*3mm Latin letters in series for a very brief duration (i.e. 22-ms). Then the examiner asks the participant to recall the stimulus for each trial, and notes if the response is correct or not.
B. Shooting-plate. This apparatus consists of a weft plate with visual stimulus (shows 3*3mm Latin letters) written on it. All stimuli are read in transparent light. The individual pieces of transparent paper are glued on cardboard cards, which had a corresponding rectangular opening. The transparent paper is encircled by a 19-mm width photographic shutter width that could open and let light get in (and allow potential visual capture of the stimulus) for a very short time (16.7 ms). The firing of the plate following the sign "Now!" was left to the patient himself, which elicited his focus on the task and prevented for attentional lapse. Then, the participant is asked to reproduce what he saw after breaks of 0, 10 and 30 s in order to gain a measure of his ability to catch and remember visual stimulus after a very-short presentation.
C. Sensory performance in the shooting-plate experiment (from Ref. [Reference Busch3]). Patients showed a significant deficit in performance across all timepoints (F1, 21=8.63, p=0.008). *p<.05, between group t-test; **p<.01.
D. Tone-matching performances (from Ref. [Reference Jonsson and Sjostedt7]). **p<.01 between groups; ***p<.001.
2.2. Auditory sensory deficits
As opposed to the visual experiment of Busch and Kraepelin in the early 1900’s, reproducible auditory studies of schizophrenia did not become practical until the introduction of commercial tape recorders in the 1950’s. In 1965, Billingberg & Jonsson investigated emotional processing in schizophrenia by testing the hypothesis that patients would misidentify neutral or friendly words as threatening [Reference Billingberg and Jonsson6]. They administered an “Intonation test”, in which single-words were presented with threatening, friendly or neutral intonation.
To control for the more basic ability to identify sounds, an auditory test (“sound effect test”) was also included in which participants had to identify a short sound (e,g.., a dentist drill, a train). As predicted, all patients demonstrated paranoid misattribution (i.e. “friendly/neutral” considered as “threatening”) significantly more than controls. However, schizophrenia patients were also impaired in the “sound effect test”, suggesting the possibility of a more basic underlying auditory deficit.
Consequently, a second study was conducted [Reference Jonsson and Sjostedt7] with an even simpler sensory control task (tone discrimination), in which tone-pairs were presented with either identical tones or tones that differed by an acoustic feature (length, intensity or pitch). In this study, there was no significant tendency for patients to misinterpret neutral words as threatening. Nevertheless, deficits were observed in the tone discrimination tasks across a range of physical features (Fig. 1D).
Although these studies were rarely cited at the time of publication, intercorrelated deficits in tone matching and auditory emotion recognition have now been extensively replicated in schizophrenia, and shown to correlate with impaired social function. Auditory sensory deficits have also been demonstrated neurophysiologically using preattentive auditory evoked potentials such as mismatch negativity (MMN), which is robustly impaired in schizophrenia and may predict conversion in clinical high-risk individuals, but is not yet available in clinical practice [Reference Javitt4, Reference Donde, Luck, Grot, Leitman, Brunelin and Haesebaert8].
3. Sensory deficits in schizophrenia today
Deficits in sensory processing are now well-established in schizophrenia, and include impairments not only in visual and auditory processing, but also in olfactory and sensorimotor systems. The renewed interest in sensory measures converges with a shift back to the initial Kraepelinian conceptualization of schizophrenia, which views schizophrenia as a “whole brain” disorder. In parallel, these theories converge with neurochemical theories focusing on neurotransmitters such as glutamate and GABA that are widely distributed throughout the brain, including within subcortical and cortical sensory regions (rev. in Ref [Reference Javitt4]).
To date, no clinical tests are available to capture these deficits. For example, widely used neurocognitive batteries such as the MATRICS Consensus Cognitive Battery (MCCB) use primarily pencil-and-paper testing, and do not evaluate sensory functions. Similarly, eye charts measure primarily ocular pathology, and are not sensitive to magnocellular system dysfunction. Similarly, routine audiometric testing evaluates the ability to detect discrete tones, but not the ability to differentiate between them, and thus is sensitive primarily to middle/inner-ear and brainstem pathology. Therefore, some sensory deficits may be misattributed to “disturbances of attention and higher interest”, as was the case during Bleuler’s time.
Until tests are available to assess sensory functions routinely within clinical settings, the degree to which sensory deficits contribute to impaired function for any given subjects cannot be easily evaluated. Nevertheless, care must be taken against assuming that because patients can read an eye chart that all aspects of visual perceptual function are intact, or that because they have normal audiometric function that all aspects of auditory function are intact. For example, it has recently been observed that schizophrenia patients with tone-matching deficits benefit from cognitive remediation only if auditory training is also included, while for patients without such deficits, no sensory-based training is required [Reference Medalia, Saperstein, Qian and Javitt9].
4. Conclusions
In summary, while descriptions of abnormal sensory processing were documented early in the history of schizophrenia, the experimental support at the time was not sufficiently robust to influence pathophysiological conceptualizations of schizophrenia. The basis for the conflicting findings between Kraepelin and Bleuler remains a historical mystery. However, Bleuler’s voice rang louder, with influential statements that championed intact sensory processing in schizophrenia holding firm until the early 1970s.
Modern literature makes it increasingly apparent that schizophrenia patients do indeed display specific sensory/perceptual-level impairments particularly within the visual and auditory sensory systems, and that such deficits may predate illness onset [Reference Javitt4, Reference Martinez, Gaspar, Hillyard, Andersen, Lopez-Calderon and Corcoran10]. At present, as at the time of Kraepelin and Bleuler, the types of deficits observed in schizophrenia are not captured by clinically available optometric and audiometric testing. However, the increasing use of computer- and tablet-based assessment in clinical care settings makes it feasible to start to implement these procedures during routine clinical care, and to consider these deficits when developing personalized treatment approaches.
Acknowledgements
We would like to acknowledge the assistance of Prof. Hans-Jürgen Moeller in manuscript preparation. Supported by grants MH49334 and MH109298 to DCJ and Fondation de l’Avenir BO-RM-18-001 to CD.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurpsy.2019.04.006.



Comments
No Comments have been published for this article.