Introduction
In most countries, cannabis is categorized as a drug of abuse, and its recreational use is strictly prohibited [Reference Room, Fisher, Hall, Lenton and Reuter1], while in others it is perceived as a benign, relatively harmless substance [Reference Marconi, Di Forti, Lewis, Murray and Vassos2,Reference Červený, Chomynová, Mravčík and van Ours3]. This antagonism could be explained in part by differences in the percentage of cannabis compounds (mainly, tetrahydrocannabinol [THC] and cannabidiol [CBD]), which have geographical implications. Traditional production of cannabis resin (hashish), which remains prevalent in the Rif (North Africa) [Reference Chouvy and Macfarlane4], could generate a 60% more potent form of cannabis [Reference Englund, Freeman, Murray and McGuire5]. However, new extraction techniques are now being used, mainly in central Europe and the United Kingdom, to produce extremely high-potency concentrates containing up to 75% THC. These include skunk, butane hash oil, and “Spice” [Reference Raber, Elzinga and Kaplan6,Reference Di Forti, Marconi, Carra, Fraietta, Trotta and Bonomo7].
Cannabis use may be considered a stressor for genetically vulnerable individuals [8–10]. Previous studies have reported that cannabis use increases the likelihood of early onset psychosis in risky individuals [11–15], and it could worsen the severity of psychotic symptoms [16–18]. Previous systematic reviews reported a twofold greater risk of developing a psychotic disorder in cannabis users (CU) than in nonusers (NU) [19–21]. In addition, cannabis use may induce acute cognitive effects that could diminish with abstinence [Reference Scott, Slomiak, Jones, Rosen, Moore and Gur22]. Studies about the biological basis of the effects of cannabis in the general population report that smoking cannabis dysregulates the endocannabinoid system, which restores homeostasis in cases of severe brain damage, such as inflammatory processes and neurocognitive impairment [Reference Bioque, Cabrera, García-Bueno, Mac-Dowell, Torrent and Saiz23]. In patients with first-episode psychosis (FEP), the various anomalies of the endogenous cannabinoid system include potentially increased levels of cannabinoids in the frontal cortex and cerebrospinal fluid even before the use of cannabis [Reference Dean, Sundram, Bradbury, Scarr and Copolov24,Reference Leweke, Giuffrida, Wurster, Emrich and Piomelli25]. Hence, changes to this atypical endocannabinoid system caused by external cannabinoid intake have been reported to be one of the main risk factors for the onset of FEP [Reference Muguruza, Lehtonen, Aaltonen, Morentin, Meana and Callado26]. A dose–response relationship has been established for this risk-factor, namely, the higher the levels of cannabis exposure, the higher the risk for onset of psychosis [Reference Marconi, Di Forti, Lewis, Murray and Vassos2,Reference Di Forti, Sallis, Allegri, Trotta, Ferraro and Stilo27,Reference Schoeler, Petros, Di Forti, Klamerus, Foglia and Ajnakina28]. Moreover, cannabis use may reduce the time to onset of psychosis [29–31] and may affect neurocognitive functioning [Reference Bogaty, Lee, Hickie and Hermens32]. The relationship between cannabis use and neurocognitive functioning in FEP is supported by a considerable body of empirical evidence. However, findings on the extent of this relationship are unclear. Several studies suggest a decrease in the executive function, verbal memory and working memory of CU with FEP [Reference Mata, Rodríguez-Sánchez, Pelayo-Terán, Pérez-Iglesias, González-Blanch and Ramírez-Bonilla33,Reference Gonzalez-Pinto, Gonzalez-Ortega, Alberich, Ruiz de Azua, Bernardo and Bioque34], whereas other studies, including one meta-analysis [Reference Yucel, Bora, Lubman, Solowij, Brewer and Cotton35], show better performance in the neurocognitive function of this group [36–39] or even absence of neurocognitive differences between CU and NU with FEP [Reference Bugra, Studerus, Rapp, Tamagni, Aston and Borgwardt40]. One of the explanations for better neurocognitive performance is that patients who were less impaired before onset of psychosis are more prone to use cannabis [Reference Yucel, Bora, Lubman, Solowij, Brewer and Cotton35]. The existence of moderators could explain some of the heterogeneity of previous results. Older age has been associated with better cognitive performance in verbal learning and verbal fluency in patients who use cannabis [Reference Bogaty, Lee, Hickie and Hermens32], and patients with a family history of psychosis performed better than patients without a family history of psychosis in verbal memory and executive function and had a higher global cognitive index [Reference Gonzalez-Pinto, Gonzalez-Ortega, Alberich, Ruiz de Azua, Bernardo and Bioque34].
This meta-analysis has the following objectives: (1) to provide a systematic review of the literature on the effects of cannabis use on neurocognitive functioning in patients with FEP; (2) to assess the effects of cannabis use on the neurocognitive functioning of FEP patients in eight domains, namely, premorbid and current intelligence quotient, attention, executive function, working memory, processing speed, verbal memory and learning, and visual memory; (3) to examine whether age at first use, duration and frequency of use, type of antipsychotic medication intake, sex, and age influenced the relationship between cannabis use and neurocognitive functioning. We hypothesized that CU with FEP would have worse neurocognitive functioning (with respect to worse attention, executive function, and verbal memory) than NU with FEP. Our second hypothesis was that a younger age at first use, a high frequency of cannabis use, and therapy with first-generation antipsychotics would be related to decreased neurocognitive functioning in CU with FEP.
Methods
Search strategy
The search strategy was based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA-P) Statement [Reference Moher, Shamseer, Clarke, Ghersi, Liberati and Petticrew41]. Articles were identified through extensive literature searches using six online electronic databases: PubMed, ScienceDirect, Web of Knowledge, Wiley Cochrane Library, PsycInfo (EBSCOHost), and SpringerLink. The search was limited to articles in English and only peer-reviewed articles were considered. The systematic peer-review of the literature was performed independently by two researchers (T.S.-G. and S.B.). A third researcher (A.C.) was assigned for those cases in which there was no agreement in order to decide whether the manuscript met the criteria for inclusion. All of the researchers had extended expertise in psychosis. The interrater agreement was 100%. The keywords used were “first episode psychosis AND neurocognition AND cannabis,” “FEP AND cognition AND cannabis,” “Cannabis AND neurocog* AND neuropsycholog* AND FEP,” “psychosis AND cognition AND cannabis,” “FEP AND IQ AND cannabis,” “psychosis & IQ & cannabis,” and “FEP AND cognit* AND cannabis.” Weekly bibliographical alerts were created in the selected databases. These remained active until the date of submission of the present manuscript to ensure that the most recently published studies were included. Reference lists from all the studies included and from published reviews on cannabis, FEP, and neurocognition were examined.
The initial search strategy yielded 4,691 studies that were screened in the electronic databases. The abstract and methods section were read. A total of 1,640 studies were excluded because of repetition, and 2,941 were excluded because they did not fit the inclusion criteria. This resulted in a final sample of 110 studies. Further examination of the full texts of the 110 studies led to the inclusion of 7 studies in the current review, with 14 independent samples and 78 effect sizes. Supplementary Material S1 shows a flow chart of the search procedure and Table 1 shows the summarized peer-reviewed data from the studies included in the meta-analysis.
Table 1. Selection of studies investigating cannabis use and neurocognitive functioning

a FEP: first-episode psychosis.
b HC: healthy controls.
c IQ: intelligence quotient.
d WAIS-III: Wechsler Adult Intelligence Scale, 3rd edition.
e WTAR: Wechsler test of adult reading.
f CU: cannabis users.
g NU: nonusers.
h FAS: verbal fluency test.
i COWAT: Controlled Oral Word Association.
j CANTAB: Cambridge Automated Neuropsychological Test Automated Battery.
k TMT-B: trail making test, part B.
l CPT: continuous performance test.
m TMT-A: trail making test, part A.
n WMS-R: Wechsler Memory Scale-Revised.
o TAVEC: Test de Aprendizaje Verbal España-Complutense (Spanish version of the California verbal learning test.
p WCST: Wisconsin Card Sorting Test Index.
q Authors of each study did not provide a global or independent score for each of the domains that were measured. See Table 2 for the correspondence between the subtest and the neuropsychological domains.
Selection criteria and data extraction
Cross-sectional and longitudinal studies were included in the systematic review when they met the following criteria: (1) diagnosis of FEP according to the Diagnostic and Statistical Manual of Mental Disorders (patients with psychotic symptoms who could have received antipsychotic treatment for less than 12 weeks); (2) comparison between CU with FEP and NU with FEP; (3) cannabis abuse or dependence with no other comorbid substance use disorder (except for the common mixture of tobacco and cannabis in the same cigarette when patients did not report independent tobacco use); (4) assessment of neuropsychological functioning based on valid and reliable tests commonly used in clinical practice; and (5) sufficient statistical data for transformation into effect sizes from the original researchers. The reasons for exclusion were as follows: (1) diagnosis of a category other than FEP within the psychosis spectrum (e.g., schizophrenia, substance-induced psychotic disorders, schizoaffective disorders); (2) studies on the effects of individual components of cannabis on cognitive functioning; (3) studies in which participants had poly-substance use disorders, even if there was a preferential use toward cannabis, given that other substances of abuse (e.g., alcohol, cocaine, and stimulants) are associated with altered cognitive performance [Reference de la Serna, Mayoral, Baeza, Arango, Andrés and Bombin42,Reference Harrell, Mancha, Martins, Mauro, Kuo and Scherer43]; (4) studies whose main neuropsychological outcomes required MRI-based assessment; (5) available data on cannabis use classified according to more than two different levels of use (e.g., NU plus 2 or more cannabis use pathways).
The search was limited to studies published during the last 10 years (2008 to July 2018). The studies included were coded according to the first author using a coding sheet. The outcome variable was coded as “neurocognitive domains.” In some cases, the neuropsychological instruments used to measure the cognitive domains were different. Therefore, we grouped the subtest into the following domains: current intelligence quotient (IQ), premorbid IQ, executive function, attention, working memory, verbal memory and learning, visual memory, and processing speed. Details on the correspondence between subtests and neuropsychological domains can be seen in Supplementary Material S2.
Other variables were coded according to whether they applied to CU with FEP or NU with FEP. The moderator variables collected in the CU group were mean age at first use, mean duration of lifetime cannabis use in years, and frequency of cannabis use, distinguishing between low frequency (two times or less per week) and high frequency (three times or more per week). Moreover, we extracted the type of antipsychotic medication (coded as first-generation or second-generation antipsychotics) and sociodemographic data such as sex, age, and country where the study was performed. Interrater agreement for the calculated effect was 100%.
Calculations and analyses
From each study, the information needed to calculate standardized mean differences (Cohen’s d) and their sampling variances was extracted. This information consisted of the means, SDs, and sample sizes of the two independent groups (CU and NU). Standardized mean differences were then corrected for bias by transforming them to Hedges’ g [Reference Thoma, Monnig, Lysne, Ruhl, Pommy and Bogenschutz44]. A positive Hedges’ g indicates that NU score higher in cognitive tests than CU.
All studies reported more than one effect size, because the independent groups were compared in several domains of neurocognitive performance (see Supplementary Material S2). Since effect sizes extracted from the same sample are related to each other, the application of classic meta-analytic techniques would violate the assumption of independent effect sizes. Violating this assumption, that is, ignoring dependency among effect sizes, can lead to a biased estimate of the standard error of the pooled effect and biased variance component estimates [Reference Hedges45]. Therefore, in order to account for dependent effect sizes, a three-level model was applied [Reference Becker, HEA and Brown46,Reference Van den Noortgate, López-López, Marín-Martínez and Sánchez-Meca47]. With the application of a three-level model, it is possible to model three different sources of variance: the sampling variance observed at Level 1 (which is known and calculated beforehand), the variance between the effect sizes belonging to the same study at Level 2 (within-study variance), and the variance between study effects at Level 3 (between-studies variance).
Log-likelihood-ratio-tests were performed to determine whether the within-study (Level 2) and between-studies (Level 3) variances were significant [Reference Van den Noortgate, López-López, Marín-Martínez and Sánchez-Meca48]. These tests compare the deviance of the full model (three-level model) with the deviance of the model where the between-studies variance has been ignored and with the deviance of a model that does not take into account the within-study variance. If the results of these two tests are statistically significant, then it can be concluded that there is significant variance at both levels. Furthermore, the percentage of variability due to systematic differences between studies, due to systematic differences within studies, and due to sampling variance was calculated using the formulae of Cheung [Reference Wibbelink and Assink49]. In order to explain these variances, moderator variables were introduced in the model one by one. Continuous moderator variables (i.e., age at first use, duration of use, percentage of low-frequency users, percentage of high-frequency users, percentage of participants taking first-generation antipsychotics, percentage of participants taking second-generation antipsychotics, percentage of men, and age) were centered to their mean for ease of interpretation. For some moderator variables (i.e., age, percentage of men, percentage of participants taking first-generation antipsychotics, and percentage of participants taking second-generation antipsychotics), the information was available for both the user group and the nonuser group. Therefore, we first performed an independent t test, and if no differences were found between CU and NU, the values of the moderator variables were averaged across the groups. However, if differences between the groups emerged, we performed a two-step analysis in which the data from moderators of the CU group were first introduced in the model, followed by the moderator referring to the NU group.
The validity of the results of the meta-analysis was threatened by possible publication bias. That is, if studies reporting nonsignificant results were censored or had never been submitted for publication, our sample of studies could be biased, as could, therefore, our results [Reference Cheung50]. In this meta-analysis, the presence of publication bias was evaluated by visually inspecting the funnel plot [Reference Rosenthal51] and by applying a three-level version of the Egger regression test [Reference Light and Pillemer52]. We also performed sensitivity analyses for outliers by excluding, one by one, effect sizes that were larger or smaller than two SDs above/below the mean.
All analyses were performed with the PROC MIXED procedure in SAS, which uses restricted maximum likelihood as the estimation method. Funnel plots were generated with R.
Results
Final sample of studies
The meta-analysis of the effect of cannabis use on neurocognitive performance in patients with FEP contained 7 independent studies (k) reporting on 78 effect sizes (m) and a total sample of 673 subjects. The sample consisted of 304 subjects in the CU group and 369 subjects in the NU group.
Overall analyses
The three-level meta-analysis resulted in a nonsignificant overall effect of −0.05 (SE = 0.15, p = 0.96, 95% confidence interval [CI; −0.39, 0.37]). The log-likelihood-ratio tests revealed that the between-studies variance was not statistically significant (χ2 = 2.30, df = 1, p = 0.13), while the within-study variance was statistically significant (χ2 = 640.2, df = 1, p < 0.001). Around 8.5% of the total variance in effect sizes was due to differences between studies, 86.7% of the total variance between effect sizes was attributed to differences within studies, and 4.8% of the observed variability was due to sampling variance.
Independent analysis of neurocognitive domains
Nonsignificant effects were observed in the analyses of each of the cognitive domains separately: (1) attention (d = −0.35, p = 0.68); (2) executive function (d = 0.16, p = 0.59); (3) premorbid IQ (d = 0.10, p = 0.72); (4) processing speed (d = 0.15, p = 0.69); (5) verbal memory and learning (d = −0.005, p = 0.98); (6) visual memory (d = −0.08, p = 0.78); and (7) working memory (d = −0.04, p = 0.90; see Table 2). Figure 1 shows the caterpillar plot where all the effect sizes are represented.
Table 2. Analyses of the effects of cannabis use on the neuropsychological performance domains
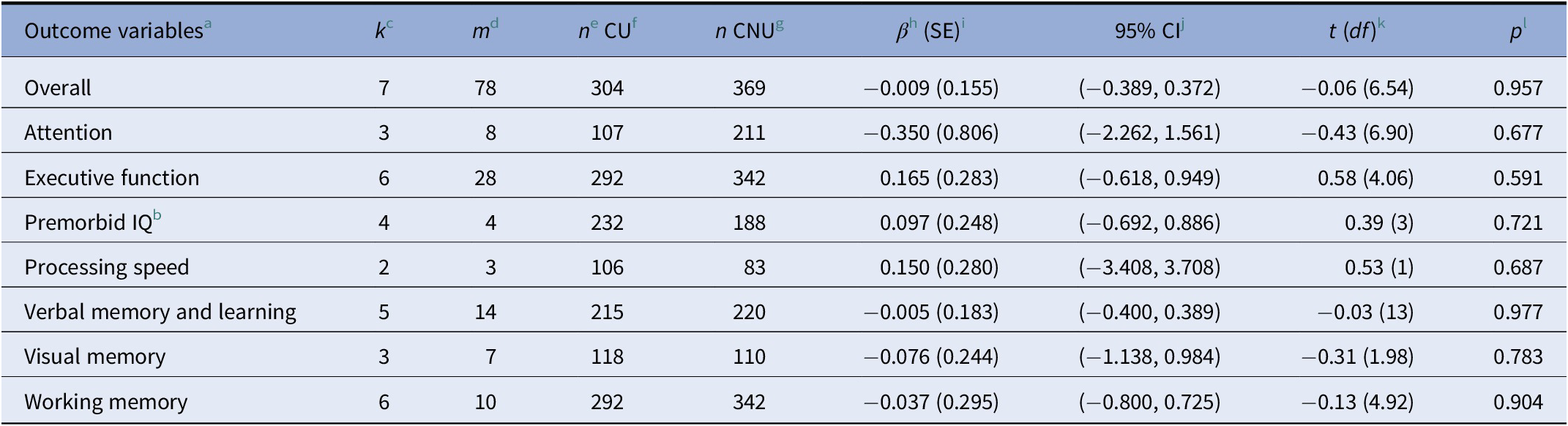
a For motor coordination and current IQ, there were not enough observations to perform the analyses.
b For premorbid IQ, a standard two-level random effects model was applied because there was only one outcome per study.
c k: number of studies that report information about the moderator variable.
d m: number of effect sizes that report information about the moderator variable.
e n: sample size.
f CU: cannabis user group.
g CNU: cannabis nonuser group.
h β: mean effect size.
i SE: standard error.
j CI: confidence interval.
k df: degrees of freedom.
l p: significant p value (p ≤ 0.005).
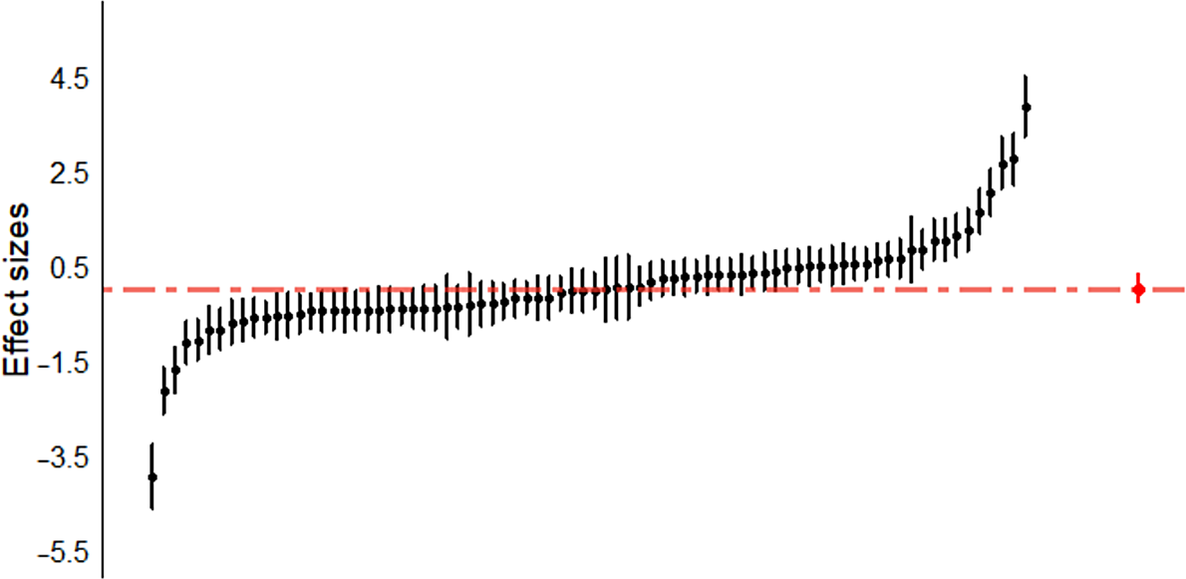
Figure 1. Caterpillar plot. Individual effect sizes (n = 78) of all of the cognitive domains (attention, executive function, processing speed, verbal memory and learning, visual memory, working memory, and premorbid IQ) are represented.
Moderator analyses
The independent t tests revealed no differences between CU and NU regarding the percentage of participants taking first- and second-generation antipsychotic medication (t = 0.314, df = 6, p = 0.37 for both analyses). Similarly, no differences were found in the mean age (t = 0.233, df = 10, p = 0.23), although there were differences across groups regarding the percentage of men (t = 5.307, df = 10, p < 0.001). This percentage was higher in CU (mean = 75.35, SD = 5.77) than in NU (mean = 52.39, SD = 8.88). Therefore, we present the effect of the percentage of men in CU and the percentage of men in NU separately.
When analyzing the effects of the moderator variables on cannabis use and neurocognitive functioning, we found that a low frequency of use led to a more positive effect size, that is, the difference in the neurocognitive functioning between CU and NU was greater, in favor of NU (F = 7.56, df = 1, 12.5, p = 0.02). Moreover, the use of first-generation antipsychotics led to a greater difference in neurocognitive functioning between the groups (F = 34.46, df = 1, 47, p < 0.001). Finally, the country where the study took place influenced the neurocognitive functioning of both groups (F = 3.75, df = 3, 70.5, p = 0.01, see Table 3).
Table 3. Moderator effect of selected variables on cannabis use and neurocognitive functioning
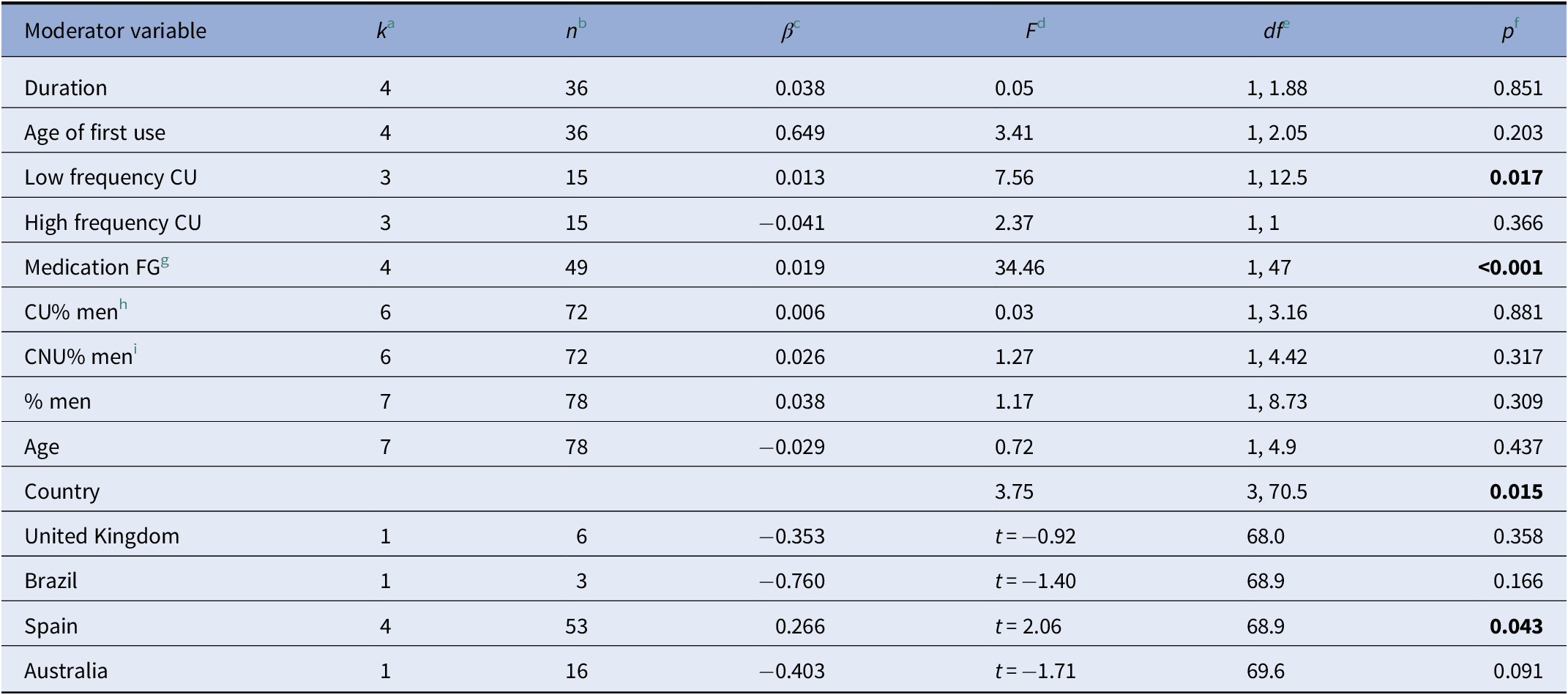
a k: number of studies that report information about the moderator variable.
b n: number of effect sizes that report information about the moderator variable.
c β: mean effect size.
d F: Value of the statistic Snedecor’s F
e df: degrees of freedom.
f p: significant p value (p ≤ 0.005).
g FG: first generation antipsychotics.
h CU: cannabis users.
i CNU: cannabis nonunsers. Probability values in bold represent significant results (p ≤ 0.05).
Sensitivity analyses
Five outliers were detected (three positive and two negative), all in the study of de la Serna et al. [Reference Egger, Davey Smith, Schneider and Minder53]. By deleting each of these outliers one by one and performing the analyses again each time, the overall effect ranged from −0.004 (95% CI [−0.44, 0.433], p = 0.98) to −0.04 (95% CI [−0.37, 0.29], p = 0.84). These results indicate that the overall estimate is quite robust.
Publication bias
Figure 2 shows the funnel plot with all of the effect sizes. The fact that effect sizes are symmetrically distributed around the mean effect seems to indicate the absence of publication bias. Furthermore, the three-level Egger regression test showed no significant association between the standard errors and their corresponding effect sizes (F = 0.01, df = 1, 14.64, p = 0.97).
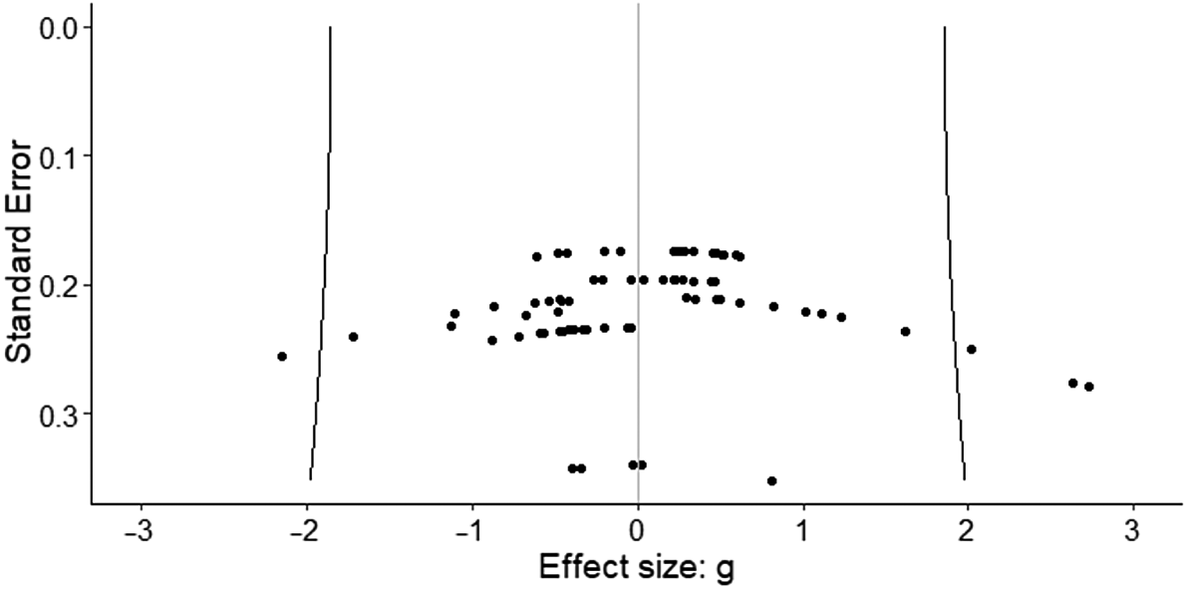
Figure 2. Funnel plot. The fact that effect sizes are symmetrically distributed around the mean effect seems to indicate the absence of publication bias.
Discussion
The results of this three-level model meta-analysis revealed no significant differences between CU and NU with respect to neurocognitive functioning. These findings are consistent with those of some previous studies, which show an absence of differences in neurocognitive functioning between CU and NU with FEP [Reference Bugra, Studerus, Rapp, Tamagni, Aston and Borgwardt40], but not with those of others [Reference Bogaty, Lee, Hickie and Hermens32]. The lack of significant differences in neurocognitive functioning between CU and NU with FEP in this study could be explained by the fact that vulnerable patients with better cognitive function can develop psychosis owing to cannabis use; this subsample can mask the deleterious effect of cannabis on cognition in other patient groups. In addition, the results can be explained by the different times of exposure to cannabis. Cumulative exposure to cannabis in a developing vulnerable adolescent brain accounts for an increased risk of onset of psychosis and, therefore, the manifestation of its symptoms [16-18], including the effects of the disease itself on neurocognitive functioning [Reference Bogaty, Lee, Hickie and Hermens32]. The present results may have been masked by the different durations of cannabis use. However, most of these results have to be interpreted with caution owing to the small number of observations (i.e., processing speed and current IQ), thus revealing the lack of sufficient power to detect a significant effect. Future research might investigate the effect of duration of cannabis exposure prior to the onset of FEP and test whether longer exposures translate into effects on neurocognitive functioning.
The analysis of the moderator variables showed that the percentage of participants who less frequently used cannabis had a more positive influence on the pooled effect, that is, the higher the number of participants who used cannabis less frequently, the better the performance of the NU in cognitive domains (and the worse the performance of users). This remarkable and counterintuitive result can be explained by differences in the underlying mechanism of psychosis associated with frequency of use for CU. Heavy, repeated cannabis use, particularly during adolescence in patients without psychosis, has been associated with adverse effects on the endocannabinoid system, especially in genetically predisposed persons [Reference Bloomfield, Hindocha, Green, Wall, Lees and Petrilli54]. Chronic and heavy use of cannabis in persons without psychosis affects cognitive domains such as memory and attention, as well as their associated brain areas [Reference Weinstein, Livny and Weizman55]. However, heavy use in patients with a very high genetic risk (family history of psychosis) is associated with better results in some cognitive domains, such as verbal learning [Reference Gonzalez-Pinto, Vega, Ibanez, Mosquera, Barbeito and Gutierrez29]. As for lower use of cannabis and psychosis, a recent study found a cluster association between poor cognition and low cannabis use in comparison with moderate and heavy use [Reference Schnakenberg Martin, Bonfils, Davis, Smith, Schuder and Lysaker56]. The endocannabinoid system restores homeostasis in brain functioning during brain damage, such as the neurocognitive impairment that is observed in patients with psychosis [Reference Bioque, Cabrera, García-Bueno, Mac-Dowell, Torrent and Saiz23]. The presence of exogenous cannabis is one of the main factors accounting for the dysregulation of the endocannabinoid system [Reference Muguruza, Lehtonen, Aaltonen, Morentin, Meana and Callado26]. As a result, the use of cannabis, even at low doses, in adolescents with a low threshold for developing psychosis after cannabis use may trigger the dysregulation of this system and accelerate the incidence of positive, negative, affective, and cognitive psychotic symptoms [16-18,Reference Bogaty, Lee, Hickie and Hermens32]. Consequently, the results of the present meta-analysis may support the finding that cannabis use acts as a risk factor for the worsening of cognitive function in a subsample of patients with a low threshold for developing FEP in CU [Reference Mata, Rodríguez-Sánchez, Pelayo-Terán, Pérez-Iglesias, González-Blanch and Ramírez-Bonilla33,Reference Cunha, Rosa, AeM, Duran, Santos and Scazufca36,Reference Leeson, Harrison, Ron, Barnes and Joyce37]. Further analysis must investigate whether cognition in this subsample improves once cannabis is no longer used.
The analysis revealed significant effects of first-generation antipsychotics and second-generation antipsychotics. Within the group of patients taking first-generation antipsychotics, the percentage of drugs increased with differences in neurocognitive functioning between CU and NU with FEP. Thus, CU scored higher in all of the neurocognitive domains despite the absence of significant results. These findings are consistent with studies on the differential antidopaminergic effects of first and second-generation antipsychotics and the disturbing effects of cannabis use on the homeostatic effects of endocannabinoids such as anandamide and 2-arachidonoylglycerol (2-AG) [Reference Giuffrida, Leweke, Gerth, Schreiber, Koethe and Faulhaber57,Reference Fernandez, Galán, Arias and Ramos58]. FEP patients present increased endocannabinoid levels in the frontal cortex and in cerebrospinal fluid even before using cannabis [Reference Dean, Sundram, Bradbury, Scarr and Copolov24,Reference Leweke, Giuffrida, Wurster, Emrich and Piomelli25]. This was mainly due to the counteracting effects of anandamide and 2-AG in patients with psychosis and in individuals at risk for psychosis who present increased levels of dopamine in the mesolimbic areas [59-61]. The endocannabinoid system increases the level of anandamide and 2-AG to naturally balance dopamine hyperactivation in mesolimbic areas [Reference Rodríguez de Fonseca, Del Arco, Martín-Calderón, Gorriti and Navarro62]. However, first-generation antipsychotics antagonize the postsynaptic dopaminergic D2 receptors, thus decreasing levels of dopamine [Reference Valenti and Grace63] and helping endocannabinoids to achieve homeostasis. This pharmacological action implies an excessive decrease in dopamine in the mesocortical circuits, thus increasing the onset of negative and cognitive symptoms. In contrast, second-generation antipsychotics interact not only with the dopamine system, but also with the serotoninergic neurotransmitter system [Reference Kuroki, Nagao and Nakahara64], thus decreasing the incidence of negative and cognitive symptoms of psychosis [Reference Giuffrida, Leweke, Gerth, Schreiber, Koethe and Faulhaber57]. Cannabis use may dysregulate both the homeostasis task of the endocannabinoid system [Reference Gardner and Vorel65,Reference Pertwee66] and worsen the pharmacological effects of antipsychotics. This result may have clinical implications for the development of patient-focused medicine, which implies the selection of second-generation antipsychotics in CU.
Our study also reveals a significant country effect. It seems that studies performed in Spain yielded a higher (positive) effect size, which was significantly different from zero, meaning that NU score higher than CU in cognitive domains. In contrast, no significant effects were found in the other countries. These results should be interpreted with caution because most of the studies that met the criteria for the present meta-analysis were carried out in Spain. Consequently, Spain is overrepresented in our data. Even so, this outcome might be explained by the differences in cannabis preparations worldwide and the use of genetically modified plants with a high THC content, such as the popular skunk or sinsemilla, which is used predominantly in Central Europe and the UK [Reference Chouvy and Macfarlane4,Reference Englund, Freeman, Murray and McGuire5,Reference Di Forti, Marconi, Carra, Fraietta, Trotta and Bonomo7]. These cannabis products have significantly decreased CBD [Reference Potter, Hammond, Tuffnell, Walker and Di Forti67], one of the several cannabinoids present in the Cannabis sativa plants. CBD has shown antipsychotic properties and preserves cognitive deterioration effects in patients with psychosis [68-70]. In conclusion, the dose, frequency, and different types of cannabis preparations may interfere with the neurocognitive functioning of CU with FEP.
The results of this study show the importance of some moderators in the cognitive skills of patients with psychosis. However, as these results are associations, we cannot infer causal relationships. In addition, heavy cannabis use in patients with chronic schizophrenia leads to worse cognitive performance than in moderate users [Reference Schnakenberg Martin, Bonfils, Davis, Smith, Schuder and Lysaker56], and this can be explained by the deleterious chronic effect of cannabis use in the long term, a finding not reported in the FEP samples studied in our meta-analysis. In addition, it is important to remember that quitting cannabis improves functionality in FEP in the long term [Reference Gonzalez-Pinto, Vega, Ibanez, Mosquera, Barbeito and Gutierrez29,Reference Gonzalez-Pinto, Gonzalez-Ortega, Alberich, Ruiz de Azua, Bernardo and Bioque34].
The strengths of the present study include the focus on a large sample of FEP patients and specific neurocognitive domains and the inclusion of studies in which patients only used cannabis. The study takes into account the shortcomings of previous systematic reviews and meta-analyses. Furthermore, the multilevel approach made it possible to include moderator variables. Therefore, we were able to assess the influence of cannabis duration and frequency of use, as well as demographic and clinical variables, on neurocognitive functioning outcomes [Reference Becker, HEA and Brown46,Reference Van den Noortgate, López-López, Marín-Martínez and Sánchez-Meca47], thus gaining more insight into interference with the neurocognitive performance of FEP patients. Our study is also subject to a series of limitations. First, the relatively small number of effect sizes in some of the neurocognitive domains analyzed could mean that the statistical power is insufficient to detect a significant effect between the groups. Second, some moderator variables, such as the country where the study was developed, were very uneven in terms of observations within categories. Other moderator variables such as, the educational level, which was described as a potential variable that could influence cognitive functioning [Reference Bernardo, Bioque, Parellada, Saiz Ruiz, Cuesta and Llerena71] and antipsychotic dosage were not included in this study. Only the study of Yücel et al. [Reference Yücel M, Lubman, Solowij, Brewer, Cotton and Conus38] comprised doses of chlorpromazine equivalents for each comparison group. Future studies in this field could consider the selection of these moderator variables to analyze its relationship with cognitive functioning in cannabis-users. Third, the consideration of antipsychotic treatment for the definition of FEP was not a homogenous criterion in each of the studies. While the studies of Cunha et al. [Reference Cunha, Rosa, AeM, Duran, Santos and Scazufca36], Yücel et al. [Reference Yücel M, Lubman, Solowij, Brewer, Cotton and Conus38], de la Serna et al. [Reference Egger, Davey Smith, Schneider and Minder53], and Moreno-Granados et al. [Reference Moreno-Granados, Ferrín, Salcedo-Marín and Ruiz-Veguilla72] did not include information regarding previous antipsychotic treatment to the enrollment in the study, the studies of Leeson et al. [Reference Leeson, Harrison, Ron, Barnes and Joyce37], Rodríguez-Sánchez et al. [Reference Rodríguez-Sánchez, Ayesa-Arriola, Mata, Moreno-Calle, Perez-Iglesias and González-Blanch39], and Mata et al. [Reference Mata, Rodríguez-Sánchez, Pelayo-Terán, Pérez-Iglesias, González-Blanch and Ramírez-Bonilla33] enrolled FEP patients who could have received neuroleptic medication for more than 12, 6, or 4 weeks, respectively. Future research may account for this limitation in order to observe if there were any cofounding effects regarding antipsychotic medication exposition at the onset of the disorder. Fourth, due to the wide exclusion criteria selected for this study, it could be considered that the sample obtained for this meta-analysis is not naturalistic. Finally, all of the studies included in the meta-analysis were cross-sectional; therefore, a longitudinal follow-up of these studies could help elucidate whether the absence of differences, the duration of psychosis, and the level of cumulative cannabis exposure all remain the same.
Conclusions
In conclusion, the current study shows that cannabis use is not related to the neurocognitive functioning of patients with FEP. It also highlights the importance of moderators. Future research is needed to understand how the neurocognitive functioning of patients with FEP is affected by dose, duration of cannabis exposure, and different types of cannabis preparations, as well as the potential interactions between antipsychotic medication and cannabis use.
Acknowledgment.
We would like to acknowledge the use of the facilities and support of Universidad Internacional de La Rioja (UNIR).
Financial Support.
This study was partially funded by UNIR Research (http://research.unir.net), Universidad Internacional de La Rioja (UNIR, http://www.unir.net), under Research Support Strategy 3 [2015–2017], Research Group “PSICONLINE General Health Psychology,” and the Ministry of Economy, Industry, and Competitiveness (MINECO) within the framework of the Subprogram Challenge-Research (Retos-Investigación) I+D+I 2017 (PSI2017-82542-R).
Conflict of Interest.
The authors report no potential conflicts of interest.
Supplementary Materials.
To view supplementary material for this article, please visit http://dx.doi.org/10.1192/j.eurpsy.2019.9.








Comments
No Comments have been published for this article.