1. Introduction
Major Depressive Disorder (MDD) is one of the leading causes of disability worldwide affecting over 300 million people. [Reference World Health Organization1] Around 30% of patients suffering from MDD do not respond to antidepressant treatment and are diagnosed with treatment resistant depression (TRD) after 2 unsuccessful courses of treatment with the appropriate dosage [Reference Rush, Trivedi and Wisniewski2, Reference Conway, George and Sackeim3]. Treatment failure has a negative effect on patients´ quality of life, is associated with increased risk for suicidal behavior and has higher economic costs for society [Reference Mrazek, Hornberger, Altar and Degtiar4]. In order to improve the efficacy of antidepressants, efforts have been made to identify biomarkers to predict treatment response, particularly within drug targets, i.e. serotonin pathways [Reference Miller, Hesselgrave and Ogden5, Reference Helton and Lohoff6]. However, the findings to date have not fulfilled expected impact possibly because an individual’s response to pharmacotherapy is multifactorial and involves a complex interplay of both genetic and environmental factors. [Reference Amare, Schubert, Klingler-Hoffmann, Cohen-Woods and Baune7]
Staging TRD has several benefits such as better predicting chances of future remission and guiding clinical treatment selection, minimizing the time that non-responders are on ineffective treatment. Various staging models of treatment resistance have been proposed. The Maudsley Staging Method for treatment resistant depression (MSM) developed by Fekadu and colleagues [Reference Fekadu, Wooderson, Markopoulou and Cleare8] has several advantages compared to previous models such as incorporating additional clinical information of duration and severity of TRD, in addition to its straightforwardness and clarity of usage. Moreover, Ruhé et al. [Reference Ruhé, van Rooijen, Spijker, FPML and Schene9] investigated several staging models for TRD and concluded that MSM has the most accurate predictive utility. This approach points towards the necessity of detecting treatment response profiles as soon as possible.
Genetic factors contribute around 35% to the disease risk in MDD, [Reference Geschwind and Flint10] and are presumed to also explain differences in treatment response [Reference Uher, Tansey and Henigsberg11]. Several genes have been associated with treatment response in MDD, including the serotonin transporter (SLC6A4), and BDNF genes (Val66Met) amongst others. [Reference Keers, Uher and Huezo-Diaz12, Reference Niitsu, Fabbri, Bentini and Serretti13] Additionally, previous studies have identified genetic variants in hepatic metabolic enzymes (CYPs) and in several polymorphisms within serotonin genes associated with response to treatment with antidepressant drugs [Reference Fabbri and Serretti14, Reference Fabbri, Crisafulli, Calabrò, Spina and Serretti15]. However, compelling evidence has identified inflammation as a promising etiopathological pathway of depression in the last 20 years. Activated inflammatory response has been detected in MDD [Reference Dantzer, O’Connor, Freund, Johnson and Kelley16]. Cytokines have been consistently shown to be elevated in depressed patients [Reference Rosenblat, Cha, Mansur and McIntyre17] and clinical similarities have been described between inflammatory diseases and MDD such as anhedonia, cognitive impairment and sleep disturbances [Reference Shariq, Brietzke, Rosenblat, Barendra, Pan and McIntyre18]. As pro-inflammatory cytokines can decrease the production of serotonin, it has been proposed that medications blocking or reducing pro-inflammatory cytokines or those increasing anti-inflammatory cytokines might have therapeutic effects in patients with MDD (see [Reference Shariq, Brietzke, Rosenblat, Barendra, Pan and McIntyre18] for a comprehensive review on targeting cytokines to reduce depressive symptoms). The underlying mechanism is that inflammatory cytokines can increase the expression and activity of monoamine transporters, the main targets of SSRIs antidepressants, [Reference Miller, Maletic and Raison19] and decrease the levels of tetrahydrobiopterin, a cofactor essential for the synthesis of serotonin [Reference Haroon, Raison and Miller20], but few studies have examined inflammatory polymorphisms as predictors of treatment response. In this regard, interleukin (IL)1-β and IL6 has shown the most promising results as linked to treatment response, with rs1143643 and rs16944 [Reference Baune, Dannlowski and Domschke21, Reference Yu YWY, Hong, Chen and Tsai22] as prime candidates of IL1-β, and rs1800795 for IL6. [Reference Carvalho, Santos and Lima23] Other inflammatory candidate genes such as IL2, IL10, IL18, tumor necrosis factor alpha (TNF-α) and interferon-gamma (IFN-γ) have received less attention, although having been previously related with major depressive disorder. [Reference Mihailova, Ivanova-Genova, Lukanov, Stoyanova and Milanova24, Reference Shelton, Claiborne and Sidoryk-Wegrzynowicz25] Taking into account that response to medication may be a polygenic and complex trait than previously hypothesized and distinct molecular mechanisms seem to modulate inflammatory pathways as stated above [Reference Malki, Tosto and Mouriño-Talín26], studies should address the role of different cytokine genetic variants, as response to medication may be as complex as the pathology. Besides, recent studies have also shown that alterations in the methylation status that regulate gene expression of these polymorphisms may contribute to disease risk and prognosis [Reference Lisoway, Zai, Tiwari and Kennedy27, Reference Bufalino, Hepgul, Aguglia and Pariante28], but few studies have examined whether inflammatory genes are differentially methylated in depression (Ryan’s study in late life depression [Reference Ryan, Pilkington, Neuhaus, Ritchie, Ancelin and Saffery29]), or the association of methylation status of such polymorphisms with treatment outcomes.
The main aim of this study was to investigate the contribution of genetic variants and methylation status in inflammatory genes to treatment response in MDD patients.
2. Methods
2.1. Sample
The study was approved by the Research Ethics Committee of Hospital Sant Pau in Barcelona and was carried out in accordance with the Declaration of Helsinki. A total of 153 psychiatric outpatients who met the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV-TR) criteria for MDD were recruited. Psychiatric comorbidities were also screened following DSM-IV-TR criteria. The diagnoses were based on clinical interviews by senior psychiatrists and double-checked through clinical reports. To be included in the study, participants had to be 18 years or older, right handed and native Catalan and/or Spanish speakers (please note that Catalan inhabitants are mostly bilingual for these two languages). The exclusion criteria were: clinically significant physical or neurological disease (brain trauma with loss of consciousness) and mental retardation (score <70 on the estimated IQ using the Vocabulary subtest of the Wechsler Adult Intelligence Scale-IV, Spanish validated version). Participants were of Caucasians of European descent and all gave informed consents after a full explanation of the study protocol and that they can withdraw at any moment. They received no financial compensation for study participation. A blood sample was obtained from each individual (10 ml) after inclusion in the study. All patients were on standard antidepressant treatment at the time of blood sampling, following the clinical guidelines of the national health system. In summary, 34% of patients were on monotherapy with SSRIs, 49% of patients were on combined treatments including SSRI or TCA plus lithium or benzodiazepines, and 17% of patients were on polytherapy, which included antidepressants (SSRIs, TCA and/or MAOIs), mood stabilizers (other than lithium) and/or antipsychotics. All medication was prescribed at therapeutic doses and for a sufficient time before changing or combining treatment strategies.
Table 1 summarizes the demographics and clinical characteristics of the study participants. A total of 56 patients had Axis I comorbidities, following the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), – affective disorder (55%), anxiety disorder (23%), psychotic disorder (11%) and schizo-affective disorder (11%). Data for smoking habits was available for 119 patients, with 22 smokers in the non-resistant group and 16 in the resistant. Detailed information of illness course and treatment response was also available. Severity of depression was assessed by the Hamilton Depression Rating Scale, 17 items (HDRS-17). [Reference Hamilton30] Illness stage was evaluated with the Maudsley Staging Method (MSM) [Reference Fekadu, Wooderson, Markopoulou and Cleare31], which included duration of index episode, symptom severity and treatment failures. This scale delivers a total score ranging from 3 to 15. For the purpose of the present study, and to better interpret the associations and to obtain odds ratios, MSM scores were also dichotomized between treatment non-response (MSM>=7; n = 62) and response (MSM<= 7; n = 91). After dividing the participants into responder and non-responder groups, the first consisted of 33 males (mean age: 44.03) and 58 females (mean age: 51.04), while in the second there were 15 males (mean age: 51.71) and 47 females (mean age: 54.73) respectively. Differences between the two groups were tested with Chi-square test for gender; and with independent t-tests for age, age at onset, number of depressive episodes, HDRS-17 and MSM scores. Expectedly, there were significant differences only in number of episodes, HDRS-17 and MSM scores (p < 0.05).
Table 1 Demographics and clinical data of the sample. The sample was divided into responders and non-responders, based on the dichotomized Maudsley Staging Method (MSM) scores as follows: MSM< = 7 were considered responders; MSM > 7, non-responders.
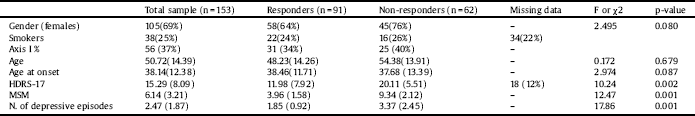
Values represent mean (SD) or otherwise specified. HDRS-17: Hamilton Depression Rating Scale, 17 items. Axis I as defined in Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). P-values considered significant at <0.05.
2.2. DNA isolation
Blood samples were systematically collected from the subjects upon admittance to the study. Genomic DNA was automatically extracted from peripheral whole-blood samples (Autopure, Qiagen, Hilden, Germany).
2.3. Genetic studies
A total of 41 Tag single nucleotide polymorphisms (SNPs) in 8 inflammatory genes (IL1-β, IL2, IL6, IL6R, IL10, IL18, TNF-α and IFN-γ) were selected for genotyping using the HapMap programme (http://www.hapmap.org). The SNP selection criteria were: minor allele frequency (MAF) over 0.05 and r2 threshold of 0.8 in Caucasians. The SNPs were analyzed by real-time PCR using OpenArray® technology on the QuantStudio™ 12 K Flex Real-Time (Applied Biosystems, Foster City, CA, USA). Standard quality controls (>95% genotyping success per individual and SNPs, Hardy-Weinberg equilibrium) were applied to the genotyping results.
2.4. Methylation analyses
Specific CpG sites in the 5′ regulatory region of selected genes (see Table 2 for details) were assessed by bisulfite-pyrosequencing. PyroMark Assay Design Software v.2.0 (QIAGEN, Hilden, Germany) was used to design the set of primers for PCR amplification and sequencing. Information on CpG sites locations and primers used is shown in Table 2. DNA bisulfite treatment and PCR amplification were performed by means of EpiTech Bisulfite kit and the PyroMark PCR kit (QIAGEN, Hilden, Germany) respectively, following the manufacturer recommendations. Pyrosequencing reactions and methylation quantification were performed in a PyroMark Q24 (QIAGEN, Hilden, Germany).
2.5. Statistical analyses
Linear and logistic regression analyses for genotype results were conducted considering MSM as the dependent variable (as continuous and binary factors respectively). A logistic regression analysis was performed for methylation results, using binary MSM distribution. Age and gender of patients were included as covariates in all analyses. Statistical analyses were performed using PLINK (version 1.07, Purcel et al., 2007). Significance was set at p < 0.05. Correction for multiple comparisons in allelic and genotype associations was carried out with false discovery rate (FDR).
3. Results
3.1. Genetic results
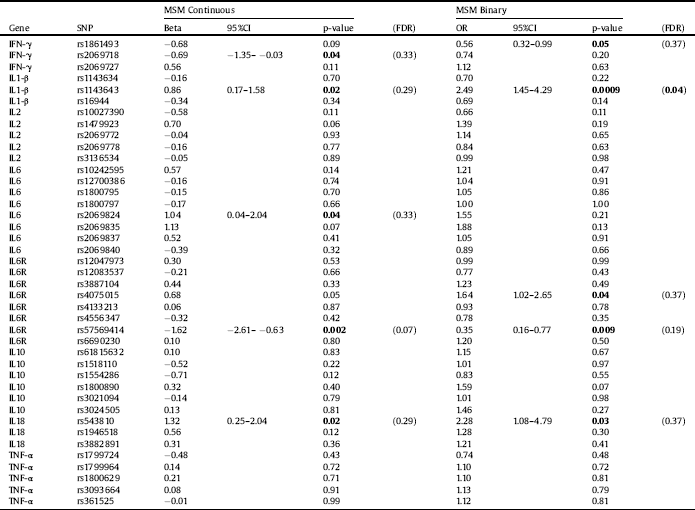
All investigated polymorphisms were in the Hardy–Weinberg equilibrium and showed a genotyping success >95%. Single marker analysis revealed uncorrected significant associations with the total MSM scores. The allelic distribution of an IL6R rs57569414 polymorphism was associated with MSM total scores (OR=-1.62; p = 0.002). Uncorrected significant associations were also found between MSM total scores and IL18 rs543810, IL1-β rs1143643, IL6 rs2069824 and IFN-γ rs2069718. Results from the allele association analysis are presented in Table 3. Analyses of genotype frequencies revealed nominal associations between the IL6 rs2069824, IL6R rs4075015, IL2 rs1479923 and IL10 rs3021094 polymorphisms and MSM scores (p values<0.05 in all comparisons). Results from the genotype analysis are displayed in Table 4. Finally, uncorrected significant associations between haplotype combinations of the IL1-β, IL6 and IL10 polymorphisms investigated were also observed (p values = 0.02, 0.03 and 0.03, respectively).
Logistic regression models (i.e., responders vs non-responders) revealed uncorrected associations between the allelic and genotype distribution of the IL1-β rs1143643 polymorphisms and response to treatment (OR = 2.49, p = 0.0009; and OR = 2.79, p = 0.002, respectively). The allele and genotype frequency distribution of the IL6R rs4075015 polymorphism were also associated with MSM as a binary variable (p = 0.04 and p = 0.03, respectively). The allele distribution of IL6R rs57569414 (p = 0.009), IL18 rs543810 (p = 0.03) and IFN-γ rs1861493 (p = 0.046) were also significantly associated with treatment resistance. Additionally, the genotype distribution of IL2 rs10027390 was nominally associated with treatment response (p = 0.05). Results from the genotype analysis are displayed in Table 4. Haplotype analyses revealed associations between allelic combinations of the genes IL1-β, IL10, IFN-γ and IL6R with treatment response (uncorrected p = 0.0006, p = 0.003, p = 0.03 and p = 0.05, respectively).
Most of the associations vanished after FDR correction. Only the allelic association of IL1-β rs1143643 with MSM scores remained significant and the genotype association of IL1-β showed a trend towards significance (see Tables 3 and 4, respectively). Haplotype analyses for allelic combinations of IL1-β and IL10 with binary MSM scores survived FDR correction (p = 0.004 and p = 0.01, respectively).
Table 2 PCR amplification and pyrosequencing’s design.

ID: identifier; F: forward; R: reverse; S: sequencing; Localization according to the accession numbers. IL: interleukin. NG_011640 (IL6), NG_012087.1 (IL6R), NG_008851 (IL1-β).
Table 3 Allele associations with the Maudsley Staging Method (MSM) scores used as a continuous or binary (responders vs. non-responders) variable. SNP: single nucleotide polymorphism. FDR: false discovery rate. Significant associations are presented in bold. IFN: interferon; IL: interleukin; TNF: tumor necrosis factor.
3.2. Epigenetic results
None of the thirteen analyzed CpG sites in the IL6, IL6R and IL1-β genes showed statistically significant differences in the methylation status when MSM score was used (p > 0.05 in all comparisons). However, when comparing the methylation percentage of treatment responders and non-responders, one of the CpG sites in the IL6R gene showed a trend towards significance (1.7 vs. 1.5, respectively; p = 0.05, uncorrected for multiple testing). Results are summarized in Table 5.
4. Discussion
The current study investigated the influence of genetic alterations in inflammatory genes in relationship with treatment response in MDD. Inflammation is thought to play a role in the pathogenesis of depression, but the effect of inflammatory pathways on treatment response is still unclear. Our findings suggest that other factors beyond the serotonergic system may be involved in treatment response in major depression. In our study, IL1-β polymorphism showed consistent association with treatment response as measured with the MSM. IL6 and IL6R polymorphisms were marginally associated, although significance did not survive correction for multiple comparisons.
The association of IL1-β rs1143643 is in agreement with a previous study by Baune and colleagues [Reference Baune, Dannlowski and Domschke21] who reported two IL1-β polymorphisms (rs1143643 and rs16944) to be related to SSRI treatment resistance. Another study by Yu and co-workers [Reference Yu YWY, Hong, Chen and Tsai22] also reported that the IL-1β rs16944 polymorphism was associated with poorer outcome after 4 weeks of treatment with fluoxetine. However, we were not able to replicate this finding in our study. This might be due to the fact that in this study patients were on a strict 4-week treatment with fluoxetine, while our patients were on a variety of antidepressants and were assessed after a long period of treatment. In addition, the different ethnicity of the participants may also account for the discrepancy. Nevertheless, we observed a significant association between IL1-β haplotype combinations and treatment response.
With regards to IL6, the current results suggest that the rs2069824 polymorphism might be associated with treatment response, although these findings should be taken cautiously as were uncorrected for multiple comparisons. In any case, the rs2069824 allelic and genotype association with MSM suggest that this variant might be a prime candidate for future investigation. We did not find association with any of the other IL6 polymorphisms investigated. Carvalho and colleagues [Reference Carvalho, Santos and Lima23] had previously reported increased risk for resistance for patients having the rs1800795-“G/G” genotype. But, methodological differences such as study design and patients’ selection could be underneath this discrepancy. In any case, both studies point towards the fact that genetic variations within cytokine genes, such as IL6 may be involved in antidepressant treatment outcomes.
Table 4 Genotype associations with the Maudsley Staging Method (MSM) scores used as a continuous or binary (responders vs. non-responders) variable. SNP: single nucleotide polymorphism. Significant associations are presented in bold. IFN: interferon; IL: interleukin; TNF: tumor necrosis factor.
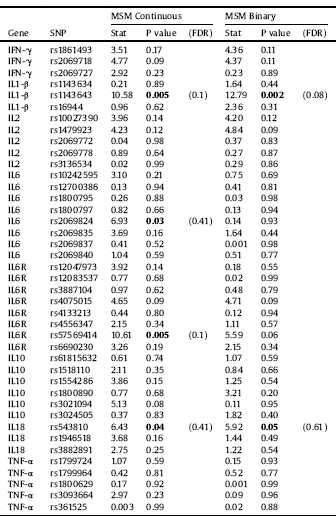
Table 5 Average of methylation percentage between non-responders (MSM > 7) and responders (MSM< = 7) in specific CpG islands of the IL6, IL6R and IL1-β genes. The analysis included age and gender as covariates. After adjusting for multiple testing (adjusted p-value) none of the differences were significant. MSM = Maudsley staging method; FC = fold change; IL: interleukin.
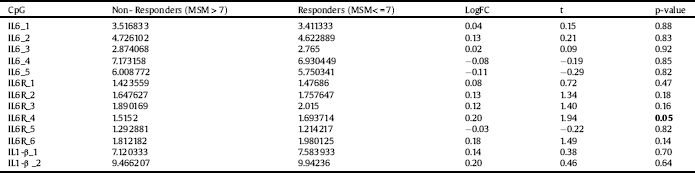
Interestingly, we also found IL6R gene to be related to treatment response -rs4075015 and rs57569414 emerged as possible predictors in both the allele and genotype analyses, together with a marginal haplotype combination-. As the pro-inflammatory signaling of IL6 depends on the soluble IL6R [Reference Hodes, Ménard and Russo32], it can be suggested that these genetic variants might play a role in this process. Our exploratory epigenetic study revealed marginal difference in IL6R methylation, with responders showing a higher methylation than non-responders, suggesting that an alteration in expression of the receptor gene may be associated with fluctuations in IL6 peripheral levels (see for example [Reference Kiraly, Horn and Van Dam33] reporting repeatedly increase plasma levels of IL6 in patients with worse treatment prognosis). Previous studies suggested that methylation changes might occur in MDD patients. An Epigenome wide study (EWAS) conducted in post-mortem frontal cortex from MDD patients revealed alterations in the methylation status of 224 regions, with differences >10%. [Reference Sabunciyan, Aryee and Irizarry34]. Recently, several studies have suggested that the methylation status of genes such as SLC6A4, NR3C1, BDNF and IL6 may constitute peripheral biomarkers for MDD. [Reference Januar, Ancelin, Ritchie, Saffery and Ryan35–Reference Roy, Shelton and Dwivedi37, Reference Ryan, Pilkington, Neuhaus, Ritchie, Ancelin and Saffery29], Uddin and co-workers [Reference Uddin, Koenen, Aiello, Wildman, De Los Santos and Galea38] reported an inverse correlation between the level of methylation of IL6 CpGs and circulating IL6 and levels in patients with lifetime depression. We observed additional associations between pro-inflammatory and anti-inflammatory variants and treatment response. Previous research has suggested that pro-inflammatory IL18 variants might play a role in MDD. Haastrup and colleagues [Reference Haastrup, Drachmann and Bock39] reported association between the IL18 rs1946518 variant and increased risk of depression in patients with past stressful life events. However, in our study we did not find any significant association with this polymorphism. Nevertheless, we observed the IL18 rs543810 allelic distribution associated with MSM when treated as total score and as binary variable, with OR > 2, although such associations did not survive FDR correction. To date, no other study has linked this polymorphism with treatment response, thus the relevance of this finding may also be spurious.
Despite the increasing interest in the inflammation theory of MDD, previous genetic research on cytokines has focused mainly on IL1-β, IL6 and IL18 variants. In light of our findings, the rest of studied cytokines, i.e., IL2, IFN-γ and IL10 were not clearly associated with treatment response in our sample of MDD, but larger studies should explore the involvement of these variants in treatment outcomes. IL10 haplotype combinations were associated with response in our study. A study by Song and colleagues [Reference Song, Halbreich, Han, Leonard and Luo40] suggested decreased levels of IL10 in depressed patients after 6 weeks of treatment and our results supports the notion that IL10 might be associated with worse treatment prognosis. With all the reserves due to the exploratory nature of the present study, these findings suggest that not only pro, but also anti-inflammatory cytokines may influence treatment response. The contribution of a single polymorphism or a haplotype to the peripheral cytokine levels is not clear, although a previous study reported a correlation between a IL1-β haplotype and a 2–3‐fold increase in the secretion levels. [Reference Hall, Perregaux and Gabel41] Our results might be in line with the previously described mechanism linking increased pro- and anti-inflammatory cytokine levels with worse tratment response. Nevertheless, we did not measure the peripheral serum levels of the investigated cytokines and therefore these conclusions remain speculative.
The present study has several limitations that need to be considered when interpreting the results. It possesses the classical limitations of a candidate genes study - the sample size is moderate and results should be confirmed in a larger independent study, as previous research exploring an already published statistically significant finding for a SNP has often failed to reproduce those findings, implying a large number of false-positive reports. Another pitfall is that it is unclear whether the SNPs have functionally significant effects on the gene or they are simply useful markers. Nonetheless, together with genome wide association studies, candidate gene studies still provide important information about disease mechanisms and the ability to predict individuals who are at risk. A second limitation is that we did not control for differences in antidepressants’ type and dose taken by participants. In any case, the naturalistic nature of the present study still provides realistic information about patients that become treatment resistant. A third possible limitation is that information about experienced childhood stressful events was not collected –a factor previously linked to aberrant methylatione. [Reference Essex, Boyce, Hertzman, Lam and Armstrong42] Finally, plasma levels of specific cytokines would have added useful information to check the hypothesis of the study. In any case, a replication of these findings is warranted in future studies.
To conclude, the current findings support that treatment response might be associated with specific genetic variants, and partly by the methylation status, of the inflammation-related gene IL1-β and, to a lesser extent, IL6 and IL6R. If confirmed, these results can provide information on additional genetic markers of response and constitute putative new targets for future novel therapies.
Conflict of interests
J.D.A received consultancy and/or lecture honoraria from Lundbeck, Pfizer, Neuraxpharm and Janssen in the last year, none of them with direct relation with this work. Thre rest of authors declare no conflict of interests related with this work.
Funding
This study was supported by an Intramural (IIBSP-2015-83), and partly funded by the European Regional Development Fund (ERDF) and by CERCA Programme (Generalitat de Catalunya). M.J.P. is funded by the Ministerio de Ciencia e Innovación of the Spanish Governement and by the Instituto de Salud Carlos III through a “Miguel Servet II” research contract (CP16/00020; PI16/00302), from the National Research Plan de I+D+I 2016-2019, co-financed by the ERDF.
Acknowledgements
We would like to thank all the patients for their selfless participation in this study.








Comments
No Comments have been published for this article.