No CrossRef data available.
Published online by Cambridge University Press: 19 July 2023
The coronavirus disease 2019 (COVID-19) pandemic continues to cause significant morbidity and mortality worldwide. Since a large portion of the world’s population is currently unvaccinated or incompletely vaccinated and has limited access to approved treatments against COVID-19, there is an urgent need to continue research on treatment options, especially those at low cost and which are immediately available to patients, particularly in low- and middle-income countries. Prior in vitro and observational studies have shown that fluoxetine, possibly through its inhibitory effect on the acid sphingomyelinase/ceramide system, could be a promising antiviral and anti-inflammatory treatment against COVID-19.
The aim of this sudy was to test the potential antiviral and anti-inflammatory activities of fluoxetine against SARS-CoV-2 in a K18-hACE2 mouse model of infection, and against several variants of concern in vitro, and test the hypothesis of the implication of ceramides and/or their derivatives hexosylceramides.
We evaluated the potential antiviral and anti-inflammatory activities of fluoxetine in a K18-hACE2 mouse model of SARS-CoV-2 infection, and against variants of concern in vitro, i.e., SARS-CoV-2 ancestral strain, Alpha B.1.1.7, Gamma P1, Delta B1.617 and Omicron BA.5.
Fluoxetine, administrated after SARS-CoV-2 infection, significantly reduced lung tissue viral titres (Figure 1) and expression of several inflammatory markers (i.e., IL-6, TNFα, CCL2 and CXCL10) (Figure 2). It also inhibited the replication of all variants of concern in vitro. A modulation of the ceramide system in the lung tissues, as reflected by the increase in the ratio HexCer 16:0/Cer 16:0 in fluoxetine-treated mice, may contribute to explain these effects (Figure 3).
Image:
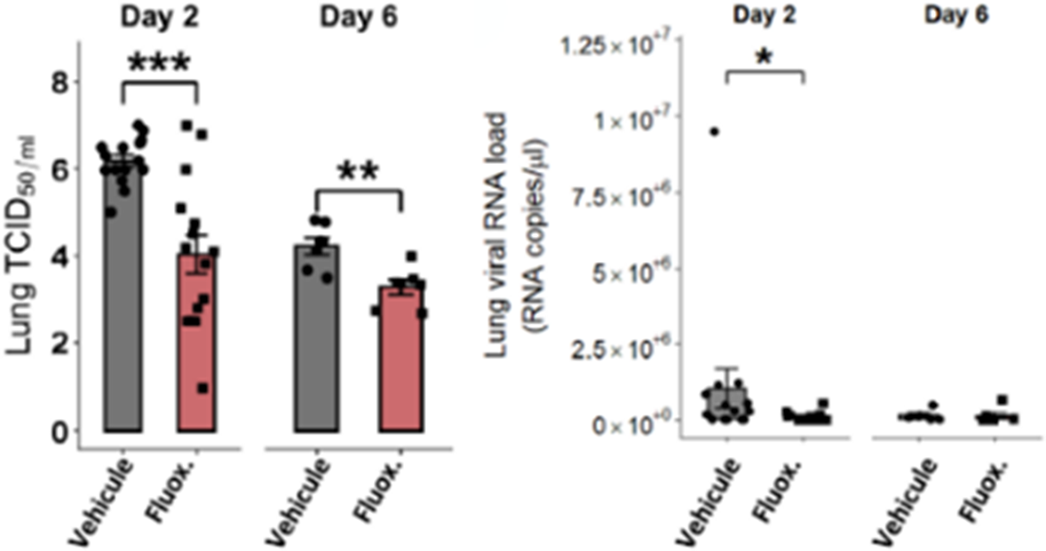
Image 2:
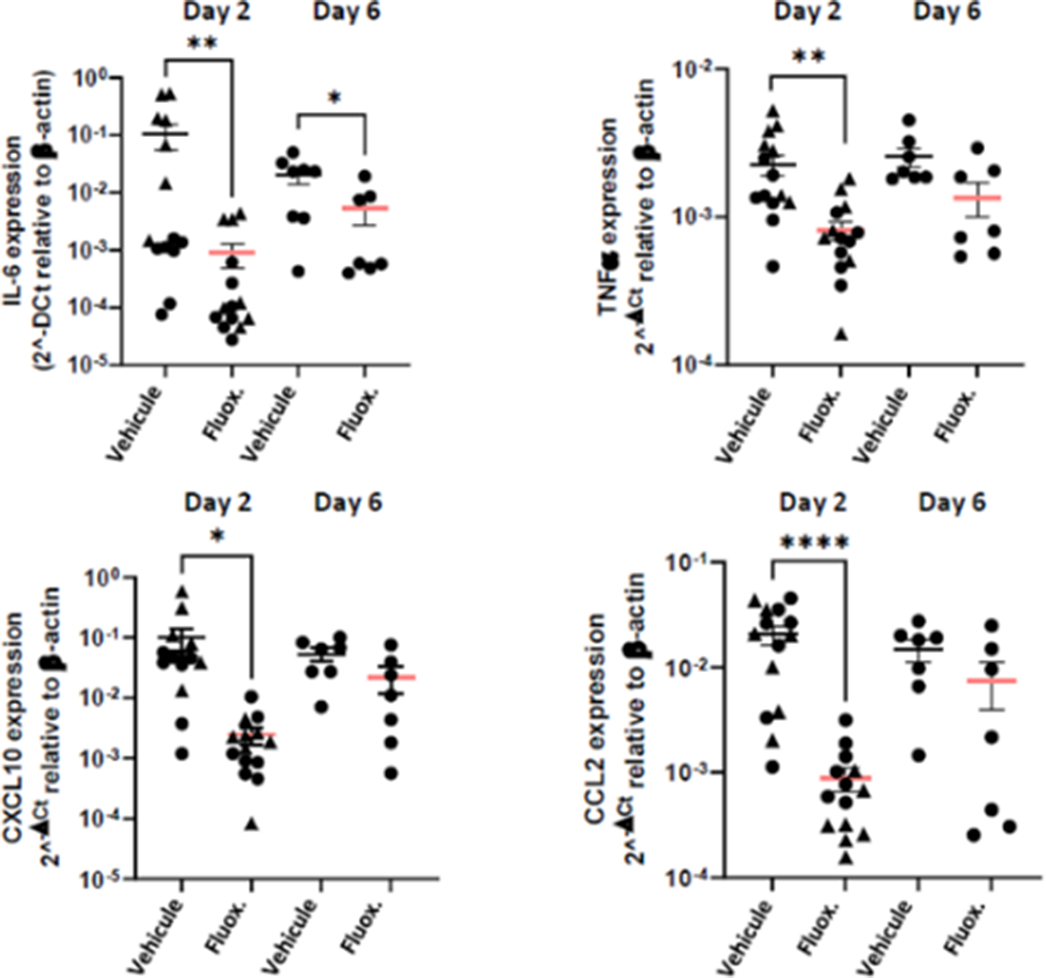
Image 3:
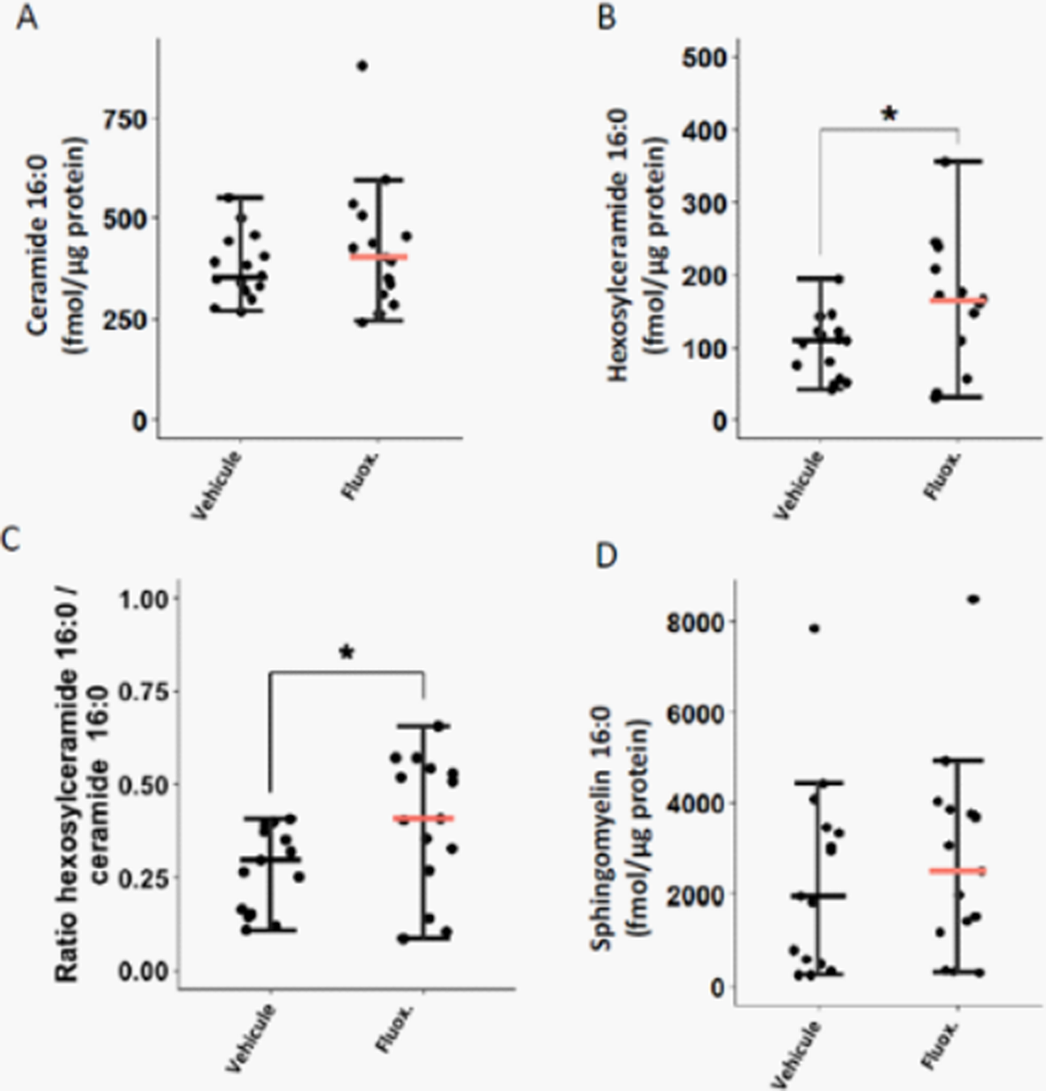
Our findings demonstrate the antiviral and anti-inflammatory properties of fluoxetine in a K18-hACE2 mouse model of SARS-CoV-2 infection, and its in vitro antiviral activity against variants of concern, establishing fluoxetine as a very promising candidate for the prevention and treatment of SARS-CoV-2 infection and disease pathogenesis.
None Declared
Comments
No Comments have been published for this article.