Introduction
Mood disorders (bipolar and depressive disorders) and anxiety disorders (separation anxiety disorder, selective mutism, specific phobias, social anxiety disorder, panic disorder, agoraphobia and generalised anxiety disorder) are complex mental disorders resulting from multiple factors such as genetic predisposition, parenting style, family environment, socioeconomic status. Fetal origins of mental disorders have been attracting increasing attention. Awareness of prenatal risk factors is crucial for prevention strategies.
Prenatal maternal health plays an important role in the subsequent mental health of offspring (O'Donnell and Meaney, Reference O'Donnell and Meaney2017). A relationship has been established between prenatal maternal psychosocial factors (psychological stress, anxiety and depression) and psychiatric disorders in offspring (Robinson et al., Reference Robinson, Lahti-Pulkkinen, Heinonen, Reynolds and Raikkonen2019; Su et al., Reference Su, D'Arcy and Meng2021). In addition to psychosocial factors, somatic diseases are also common among pregnant women. A cohort study including more than 1.3 million childbirths showed that the prevalence of maternal chronic diseases during pregnancy was 15.76%, with somatic diseases accounting for about 70% (Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Beck-Nielsen and Norgard2016). In the context of somatic diseases, hormones, metabolites, cytokines and nutrients are altered, which may implicate an adverse intrauterine environment and affect fetal neurodevelopment (Mac Giollabhui et al., Reference Mac Giollabhui, Breen, Murphy, Maxwell, Cohn, Krigbaum, Cirillo, Perez, Alloy, Drabick and Ellman2019; Nattero-Chavez et al., Reference Nattero-Chavez, Luque-Ramirez and Escobar-Morreale2019; Lu-Culligan and Iwasaki, Reference Lu-Culligan and Iwasaki2020). Furthermore, some maternal somatic diseases alter the normal composition of breast milk (Tekin Guler et al., Reference Tekin Guler, Koc, Kara Uzun and Fisunoglu2021) and severe somatic diseases could influence family environment. These could contribute to the risk of offspring mood/anxiety disorders.
Accumulating high-quality cohort studies have assessed the impact of prenatal maternal somatic diseases on mood and anxiety disorders in offspring, but the results are not consistent. To our knowledge, there have been no systematic review and meta-analysis assessing the risk of offspring mood and anxiety disorders in the context of prenatal maternal somatic diseases. The importance of prenatal maternal somatic diseases for offspring mood and anxiety disorders may be overlooked or undervalued. The relationships between them merit further investigation. The results could provide valuable information for exploring underlying mechanisms of mood and anxiety disorders, individualised risk prediction models, and targeted interventions during or before pregnancy. To fill this gap, this systematic review and meta-analysis aimed to assess the risk of offspring mood and anxiety disorders in the context of prenatal maternal somatic diseases.
Methods
This study was conducted in accordance with Preferred Reporting Items for Systematic Reviews (PRISMA) (Moher et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2009). PRISMA checklist was presented in online Supplementary materials. The protocol was registered in PROSPERO (CRD42021241441).
Search strategy
We searched the following databases for relevant literature written in English from their inception until 31 August 2021: Embase (including Embase, MEDLINE, PubMed-not-MEDLINE), PsycARTICLES (via EBSCO) and PsycINFO (via EBSCO). The search strategy consisted of relevant Medical Subject Heading (MeSH) terms, Emtree term-exploded, keywords and word variants. The detailed search strategies for each database were fully described in online Supplementary Table S1. In addition, we searched the reference lists of all included studies, related reviews for further potential studies.
Inclusion and exclusion criteria
Articles were eligible if they met the following criteria: (1) exposures: offspring were exposed to maternal somatic diseases in utero (somatic diseases diagnosed during pregnancy or chronic somatic diseases diagnosed before pregnancy; somatic diseases included all non-psychiatric diseases), (2) control group(s): had a comparison group(s) without the exposure(s), (3) outcomes: included mood or anxiety disorders diagnosed according to any recognised diagnostic criteria or self-report (mood disorders included bipolar and depressive disorders; anxiety disorders included separation anxiety disorder, selective mutism, specific phobias, social anxiety disorder, panic disorder, agoraphobia and generalised anxiety disorder), (4) statistical indicators were provided to examine the effect of prenatal maternal somatic diseases on mood or anxiety disorders in offspring, (5) study design: cohort studies, including population-based cohort studies and registry-based studies.
Articles were excluded if they met the following criteria: (1) exposed group was mixed with maternal somatic diseases diagnosed postpartum; (2) reviews, meta-analyses, abstracts or conference proceedings.
When there were multiple groups of useful data in one article, only the data derived from the group with the largest sample size or the most severe exposure (e.g. in moderate and severe obesity, the data of severe obesity were pooled) were selected for the meta-analysis. In addition, we did not delete articles that presented overlapping samples as they included different maternal somatic diseases or outcomes, but only the data from the largest sample size was used in the meta-analysis.
Selection of the studies
After removing duplications, titles and abstracts were reviewed independently by two researchers for initial screening, and then full text. Any disagreement was resolved through group discussions. Endnote was used as the bibliographic software.
Data extraction and quality assessment
Two researchers independently extracted data. Any disagreement was resolved through group discussions. The following data were extracted: author, year of publication, the country where the study was conducted, sample and source, exposure and measure, outcome and measure, adjusted confounders, and measure of association. All included articles were assessed in terms of methodological quality according to the Newcastle–Ottawa Quality Assessment Scale (NOS) for cohort studies. NOS consists of eight items across three domains: selection (four items, each with one star), comparability (one item, with a maximum of two stars) and outcome (three items, each with one star). Studies were graded as good quality (7–9 stars), fair (4–6 stars) and poor (0–4 stars).
Statistical analysis
The method was based on the relative risk (RR) with 95% confidence intervals (CIs) obtained in each study. If the RR was not reported, the hazard ratio (HR)/odds ratio (OR) is considered to be approximately equal to RR. RRs fully adjusted were preferentially pooled in our analyses. Cochrane Q test and I 2 statistics were used to evaluate the heterogeneity. A fixed-effects model was adopted when I 2 < 50%, otherwise, a random-effects model was used. Funnel plot, trim and fill method and Egger test were used to detect potential publication bias. To assess the stability of the meta-analysis, sensitivity analysis was performed. Sensitivity analysis was performed by excluding studies one by one to explore the impact of each study on the overall results. Furthermore, analyses were stratified according to maternal somatic diseases and follow-up duration (or offspring's age at diagnosis). Quantitative meta-analysis was conducted for an outcome when more than one study presented relevant data. The reason why we adopted stratified analysis rather than traditional subgroup analysis was that the former can make better use of the data in our study. Analyses were performed with Stata 16.0.
Results
In total, 21 articles were eligible for inclusion (Pang et al., Reference Pang, Syed, Fine and Jones2009; Tuovinen et al., Reference Tuovinen, Raikkonen, Pesonen, Lahti, Heinonen, Wahlbeck, Kajantie, Osmond, Barker and Eriksson2012, Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014; Robinson et al., Reference Robinson, Zubrick, Pennell, Van Lieshout, Jacoby, Beilin, Mori, Stanley, Newnham and Oddy2013, Reference Robinson, Ghassabian, Sundaram, Trinh, Bell, Mendola and Yeung2020a, Reference Robinson, Ghassabian, Sundaram, Trinh, Lin, Bell and Yeung2020b; Betts et al., Reference Betts, Salom, Williams, Najman and Alati2015; Kingston et al., Reference Kingston, Heaman, Brownell and Ekuma2015; Svahn et al., Reference Svahn, Hargreave, Nielsen, Plessen, Jensen, Kjaer and Jensen2015; Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Beck-Nielsen and Norgard2018a, Reference Jolving, Nielsen, Kesmodel, Nielsen, Norgard and Beck-Nielsen2018b; Rochat et al., Reference Rochat, Houle, Stein, Pearson and Bland2018; Dachew et al., Reference Dachew, Scott, Mamun and Alati2019, Reference Dachew, Scott, Betts, Mamun and Alati2020; Lydholm et al., Reference Lydholm, Kohler-Forsberg, Nordentoft, Yolken, Mortensen, Petersen and Benros2019; Momen et al., Reference Momen, Ernst, Arendt, Olsen, Li, Gissler and Ramlau-Hansen2019; Wu et al., Reference Wu, Dalman, Karlsson, Lewis, Osborn, Gardner and Hayes2019; Chen et al., Reference Chen, Kong, Piltonen, Gissler and Lavebratt2020; Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020; Wang et al., Reference Wang, Rolls, Du, Du, Yang, Li, Li, Cheng and Feng2020, Reference Wang, He, Miao, Yu, Liu, Zhang, Li and Li2021). A summary of the study selection process was presented in Fig. 1. Table 1 shows the general characteristics of the included studies. More than 5.7 million offspring were involved, and the sample size ranged from 778 to 2 412 721 participants. Twelve maternal somatic diseases were involved, infections (bacterial and viral infections), hypertensive disorders (chronic hypertension, gestational hypertension and preeclampsia), diabetes (type 2 diabetes, insulin-treated pregestational diabetes and gestational diabetes), thyroid diseases (Graves' disease and Hashimoto's thyroiditis), obesity [body mass index (BMI) ⩾ 30], polycystic ovary syndrome (PCOS and hirsutism), infertility, cancer, asthma, rheumatoid arthritis, hyperemesis gravidarum, migraine. According to the Newcastle–Ottawa Scale, 15 studies were graded as good quality and six were graded as fair quality (online Supplementary Table S2).
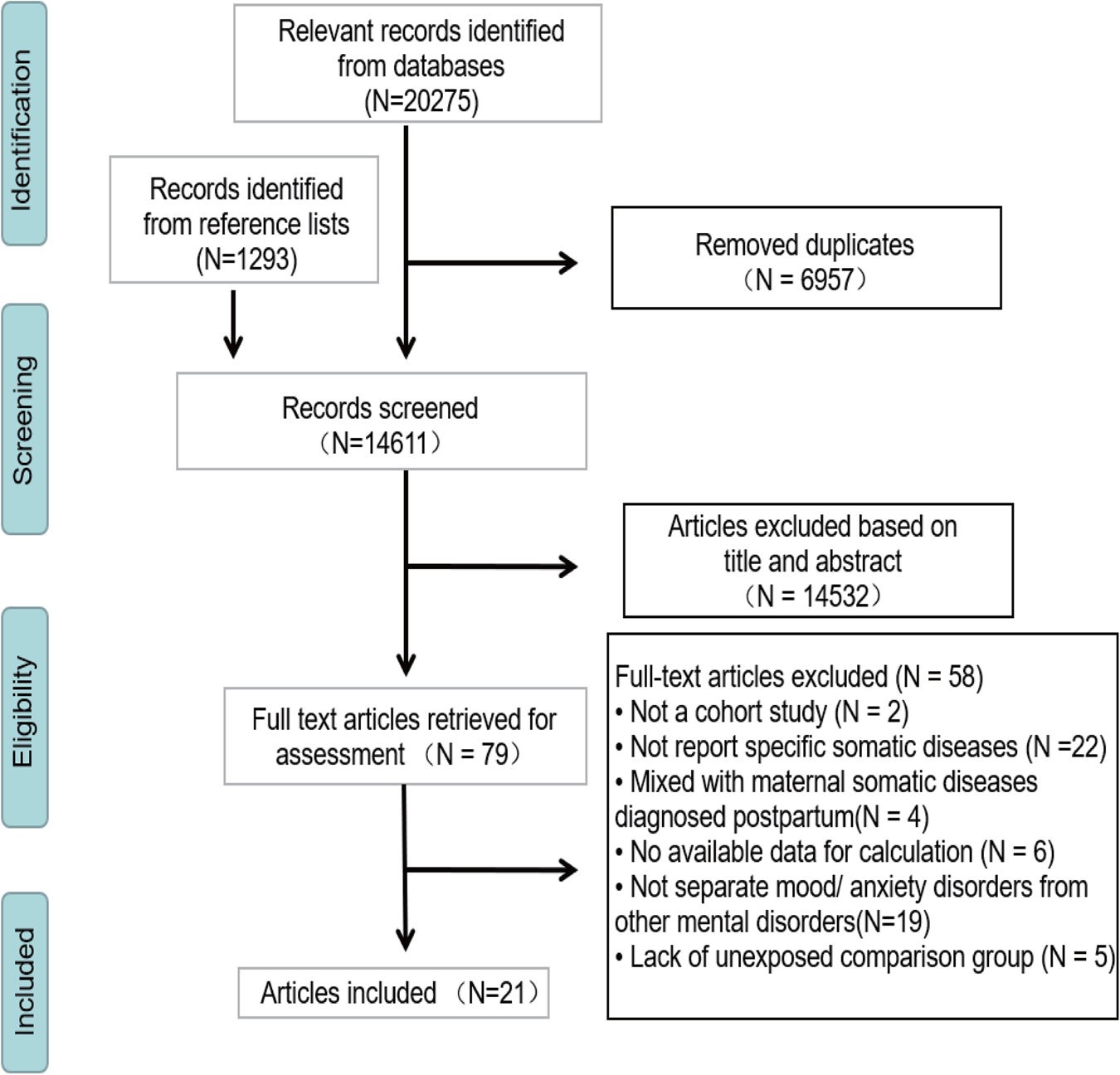
Fig. 1. Study selection process.
Table 1. Characteristics of included studies
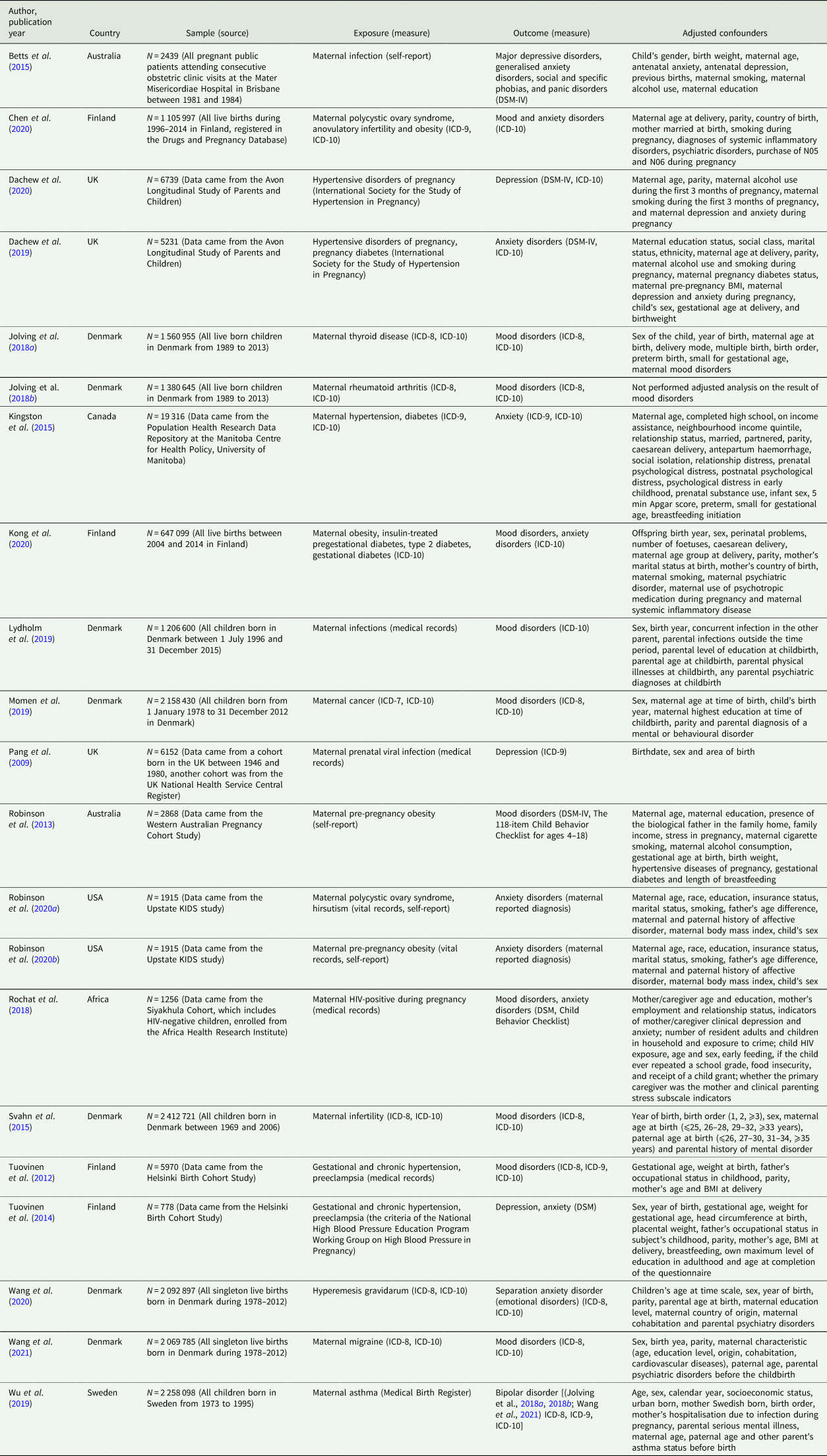
ICD, International Classification of Diseases of the World Health Organization; DSM, Diagnostic and Statistical Manual of Mental Disorders; BMI, body mass index; N05 (antipsychotics, anxiolytics, hypnotics and sedatives), N06 (antidepressants, psychostimulants and nootropics).
The risk of offspring mood disorders in the context of prenatal maternal somatic diseases
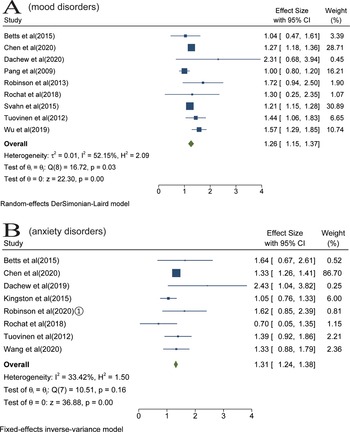
We identified 16 studies that examined the association of maternal somatic diseases with mood disorders (Pang et al., Reference Pang, Syed, Fine and Jones2009; Tuovinen et al., Reference Tuovinen, Raikkonen, Pesonen, Lahti, Heinonen, Wahlbeck, Kajantie, Osmond, Barker and Eriksson2012, Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014; Robinson et al., Reference Robinson, Zubrick, Pennell, Van Lieshout, Jacoby, Beilin, Mori, Stanley, Newnham and Oddy2013; Betts et al., Reference Betts, Salom, Williams, Najman and Alati2015; Svahn et al., Reference Svahn, Hargreave, Nielsen, Plessen, Jensen, Kjaer and Jensen2015; Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Beck-Nielsen and Norgard2018a, Reference Jolving, Nielsen, Kesmodel, Nielsen, Norgard and Beck-Nielsen2018b; Rochat et al., Reference Rochat, Houle, Stein, Pearson and Bland2018; Lydholm et al., Reference Lydholm, Kohler-Forsberg, Nordentoft, Yolken, Mortensen, Petersen and Benros2019; Momen et al., Reference Momen, Ernst, Arendt, Olsen, Li, Gissler and Ramlau-Hansen2019; Wu et al., Reference Wu, Dalman, Karlsson, Lewis, Osborn, Gardner and Hayes2019; Chen et al., Reference Chen, Kong, Piltonen, Gissler and Lavebratt2020; Dachew et al., Reference Dachew, Scott, Betts, Mamun and Alati2020; Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020; Wang et al., Reference Wang, He, Miao, Yu, Liu, Zhang, Li and Li2021). Data of seven studies were not be pooled because they used overlapping populations (Tuovinen et al., Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014; Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Beck-Nielsen and Norgard2018a, Reference Jolving, Nielsen, Kesmodel, Nielsen, Norgard and Beck-Nielsen2018b; Lydholm et al., Reference Lydholm, Kohler-Forsberg, Nordentoft, Yolken, Mortensen, Petersen and Benros2019; Momen et al., Reference Momen, Ernst, Arendt, Olsen, Li, Gissler and Ramlau-Hansen2019; Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020; Wang et al., Reference Wang, He, Miao, Yu, Liu, Zhang, Li and Li2021). The meta-analysis of nine studies suggested that the risk of offspring mood disorders increased in the context of prenatal maternal somatic diseases, with a pooled RR of 1.26 (95% CI 1.15–1.37) based on a random-effects model. High heterogeneity was observed (I 2 = 52%, p < 0.05) (Fig. 2a).
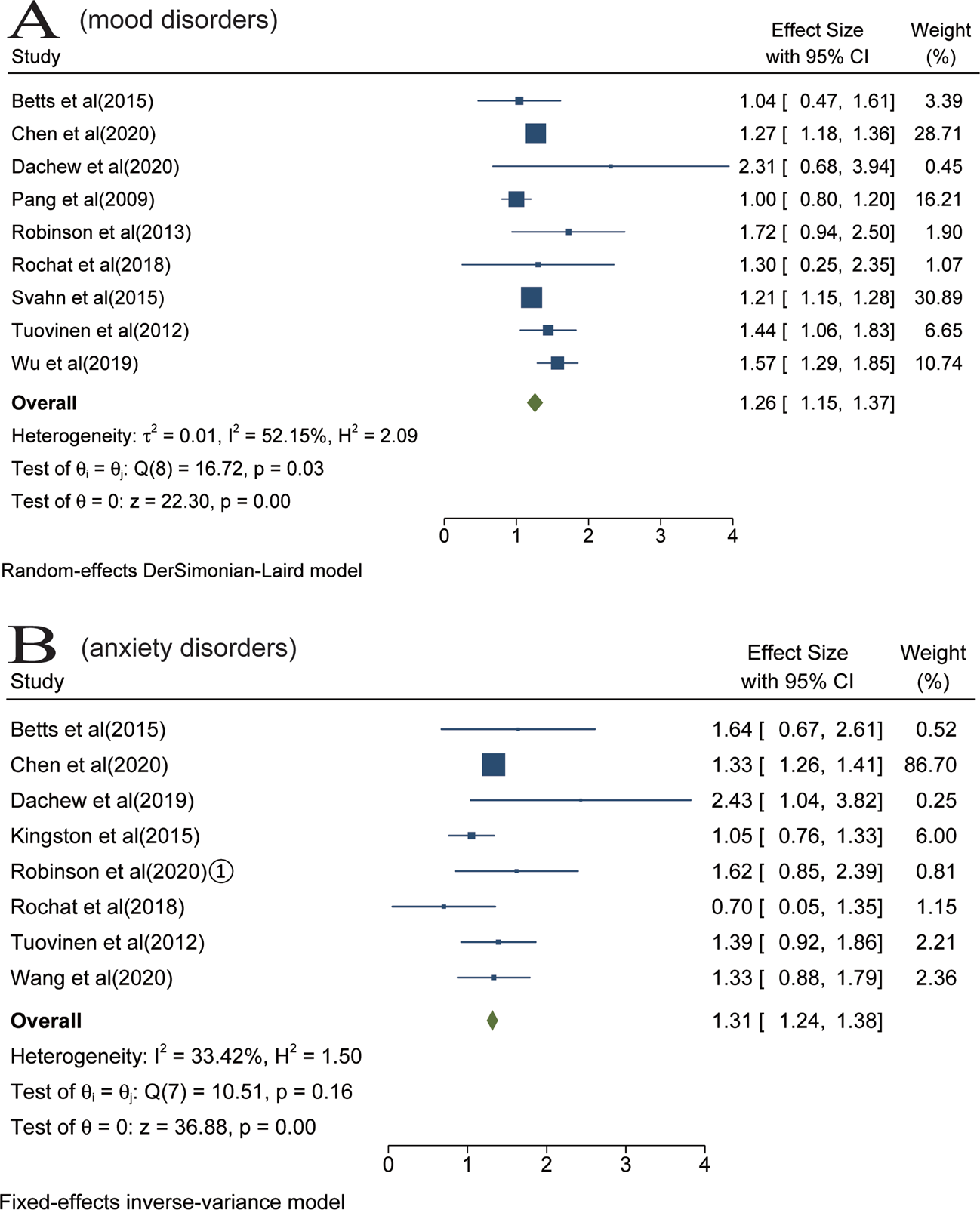
Fig. 2. Results of overall meta-analysis [Forest plot presenting combined effect estimates; ES, effect size (relative risk)]. (a) The risk of offspring mood disorders in the context of prenatal maternal somatic diseases. (B) The risk of offspring anxiety disorders in the context of prenatal maternal somatic diseases.
Analyses were stratified according to maternal somatic diseases. Pooled effect of two studies showed that maternal obesity was significantly associated with offspring mood disorders (RR = 1.92; 95% CI 1.72–2.11) (Fig. 3a). In the meta-analysis of two studies, maternal hypertensive disorders increased the risk of offspring mood disorders (RR = 1.49; 95% CI 1.11–1.86) (Fig. 3b). Pooled effect of two studies suggested that the risk of offspring mood disorders increased in the context of maternal infertility (RR = 1.26; 95% CI 1.14–1.39) (Fig. 3c). The meta-analysis of four studies found that there was no statistical association between prenatal maternal infections and offspring mood disorders (RR = 1.04; 95% CI 0.97–1.12) (Fig. 3d).
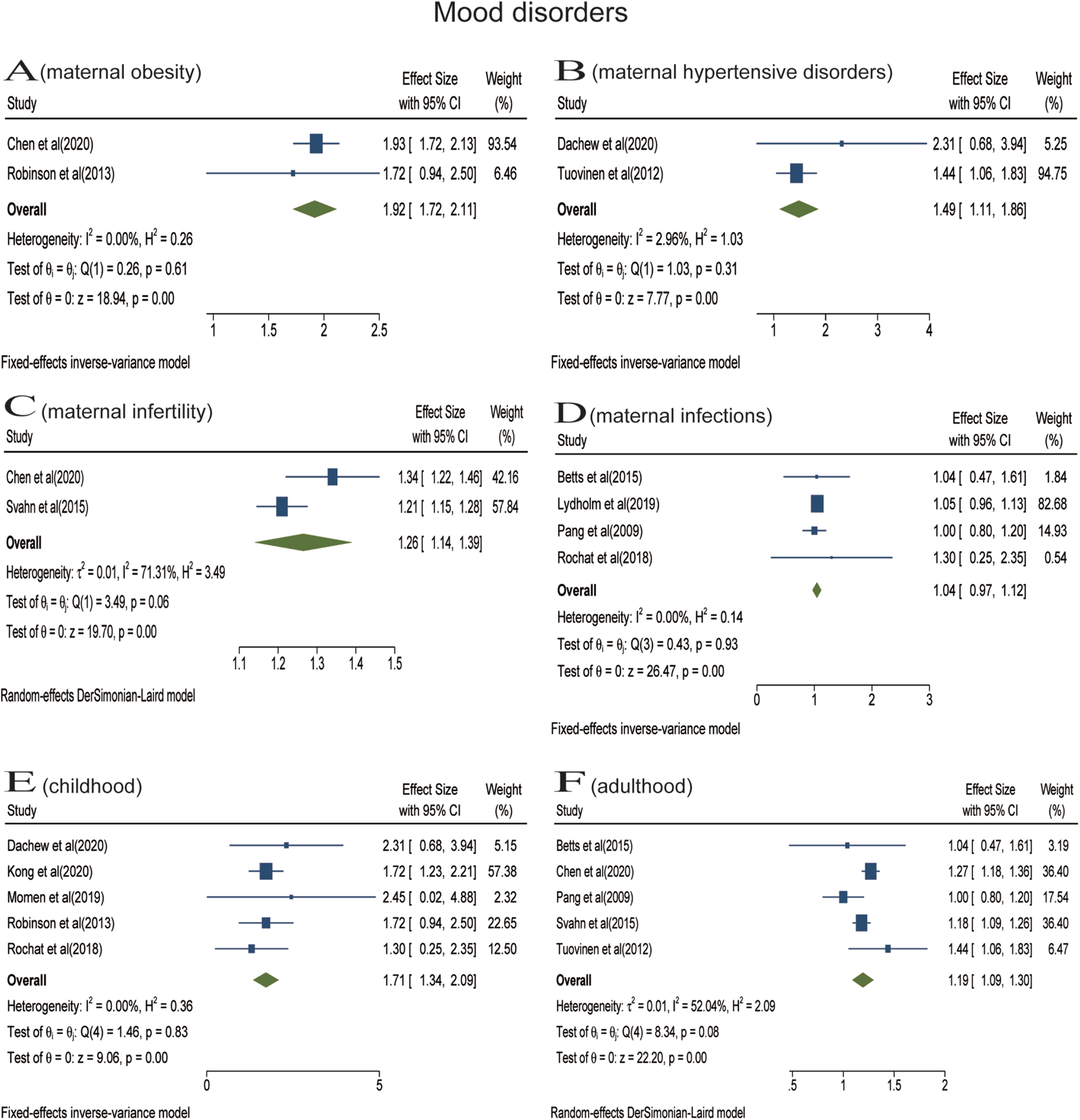
Fig. 3. Stratified analyses of mood disorders [Forest plot presenting combined effect estimates; ES, effect size (relative risk)]. (a) The association between maternal obesity and mood disorders in offspring. (b) The association between maternal hypertensive disorders and mood disorders in offspring. (c) The association between maternal infertility and mood disorders in offspring. (d) The association between maternal infections and mood disorders in offspring. (e) The association between maternal somatic diseases and mood disorders in childhood. (f) The association between maternal somatic diseases and mood disorders in adulthood.
In the stratified analyses, we did not perform meta-analyses for maternal PCOS, cancer, asthma, diabetes, thyroid diseases and rheumatoid arthritis due to the limited number of eligible studies. These studies reported that prenatal exposure to maternal PCOS (Chen et al., Reference Chen, Kong, Piltonen, Gissler and Lavebratt2020), diabetes with BMI ⩾ 35, cancer (Momen et al., Reference Momen, Ernst, Arendt, Olsen, Li, Gissler and Ramlau-Hansen2019), asthma (Wu et al., Reference Wu, Dalman, Karlsson, Lewis, Osborn, Gardner and Hayes2019) and migraine (Wang et al., Reference Wang, He, Miao, Yu, Liu, Zhang, Li and Li2021) elevated the risk of mood disorders, while maternal diabetes with BMI < 30 (Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020), thyroid diseases (Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Norgard and Beck-Nielsen2018b) and rheumatoid arthritis (Jolving et al., Reference Jolving, Nielsen, Kesmodel, Nielsen, Beck-Nielsen and Norgard2018a) were not associated with mood disorders.
Analyses were also stratified according to follow-up duration (or offspring's age at diagnosis), including childhood (aged < 18 years) and adulthood (aged ⩾ 18 years). In the meta-analysis of five studies, prenatal maternal somatic diseases were associated with the increased risk for mood disorders in childhood (RR = 1.71; 95% CI 1.34–2.09) (Fig. 3e). The meta-analysis including five studies found that prenatal maternal somatic diseases increased the risk of mood disorders in adulthood (RR = 1.19; 95% CI 1.09–1.30) (Fig. 3f). Only one article reported the impact on older offspring (aged 69.3 ± 3.1 years) and found that maternal hypertensive disorders during pregnancy (HDP) increased the risk of offspring depressive disorders seven decades later (Tuovinen et al., Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014).
The risk of offspring anxiety disorders in the context of prenatal maternal somatic diseases
Ten studies examined the association between maternal somatic diseases and anxiety disorders (Tuovinen et al., Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014; Betts et al., Reference Betts, Salom, Williams, Najman and Alati2015; Kingston et al., Reference Kingston, Heaman, Brownell and Ekuma2015; Rochat et al., Reference Rochat, Houle, Stein, Pearson and Bland2018; Dachew et al., Reference Dachew, Scott, Mamun and Alati2019; Chen et al., Reference Chen, Kong, Piltonen, Gissler and Lavebratt2020; Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020; Robinson et al., Reference Robinson, Ghassabian, Sundaram, Trinh, Bell, Mendola and Yeung2020a, Reference Robinson, Ghassabian, Sundaram, Trinh, Lin, Bell and Yeung2020b; Wang et al., Reference Wang, Rolls, Du, Du, Yang, Li, Li, Cheng and Feng2020). Data of two studies were not be pooled because they used overlapping populations (Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020; Robinson et al., Reference Robinson, Ghassabian, Sundaram, Trinh, Lin, Bell and Yeung2020b). The meta-analysis of eight studies suggested that the risk of anxiety disorders increased in the context of prenatal maternal somatic diseases, with a pooled RR of 1.31 (95% CI 1.24–1.38) based on a fixed-effects model. There was no evidence of significant heterogeneity among the studies (I 2 = 33%, p > 0.05) (Fig. 2b).
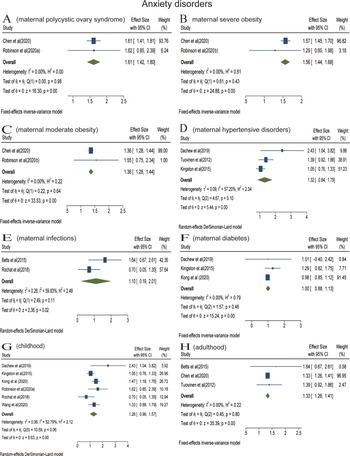
Analyses were stratified according to maternal somatic diseases. Pooled effect of two studies showed that the risk of offspring anxiety disorders significantly increased in the context of maternal PCOS (RR = 1.61; 95% CI 1.42–1.80) (Fig. 3a). In the meta-analysis of two studies, maternal severe obesity (BMI ⩾ 35) increased the risk of offspring anxiety disorders (RR = 1.56; 95% CI 1.44–1.68) (Fig. 3b). Pooled effect of two studies suggested that maternal moderate obesity (30 ⩽ BMI < 35) was associated with offspring anxiety disorders (RR = 1.36; 95% CI 1.28–1.44) (Fig. 3c). The meta-analysis of three studies found that there was no statistical association between prenatal maternal hypertensive disorders and offspring anxiety disorders (RR = 1.32; 95% CI 0.84−2.11) (Fig. 3d). The meta-analysis including two studies found that prenatal maternal infections did not increase the risk of offspring's anxiety disorders (RR = 1.10; 95% CI 0.19–2.01) (Fig. 3e). The meta-analysis did not find a statistical association between prenatal maternal diabetes and anxiety disorders of offspring among three studies (RR = 1.00; 95% CI 0.88–1.13) (Fig. 3f).
In the stratified analyses, we did not perform a meta-analysis for maternal infertility and hyperemesis gravidarum due to the limited number of eligible studies. One study reported a significant association between maternal infertility and anxiety disorders of offspring (Chen et al., Reference Chen, Kong, Piltonen, Gissler and Lavebratt2020), the other found no significant association between maternal hyperemesis gravidarum and separation anxiety disorder (emotional disorders) in offspring (Wang et al., Reference Wang, Rolls, Du, Du, Yang, Li, Li, Cheng and Feng2020).
Analyses were also stratified according to follow-up duration (or offspring's age at diagnosis). In the meta-analysis of five studies, prenatal maternal somatic diseases were not associated with offspring anxiety disorders in childhood (RR = 1.28; 95% CI 0.99–1.57) (Fig. 3g). The meta-analysis including three studies found that prenatal maternal somatic diseases increased the risk of anxiety disorders in adulthood (RR = 1.33; 95% CI 1.26–1.41) (Fig. 3h). Only one article reported the impact on older offspring and found that maternal HDP increased the risk of anxiety (Tuovinen et al., Reference Tuovinen, Aalto-Viljakainen, Eriksson, Kajantie, Lahti, Pesonen, Heinonen, Lahti, Osmond, Barker and Raikkonen2014).
Publication bias and sensitivity analysis
Due to the small number of studies after stratified analyses, funnel plot and Egger test were only calculated for the main results of mood and anxiety disorders. Although no evidence of publication bias was found based on Egger test (p > 0.05), the asymmetric funnel plot suggested the possibility of publication bias (online Supplementary Fig. S1). The ‘trim and fill’ method was then used to recalculate the pooled results. The adjusted pooled effect of mood disorders exhibited a similar trend with two potential studies filled (RR = 1.24; 95% CI 1.13–1.36) (online Supplementary Fig. S1). The result for anxiety disorders was not altered with two potential studies filled (RR = 1.24; 95% CI 1.13–1.36) (Fig. 4d).
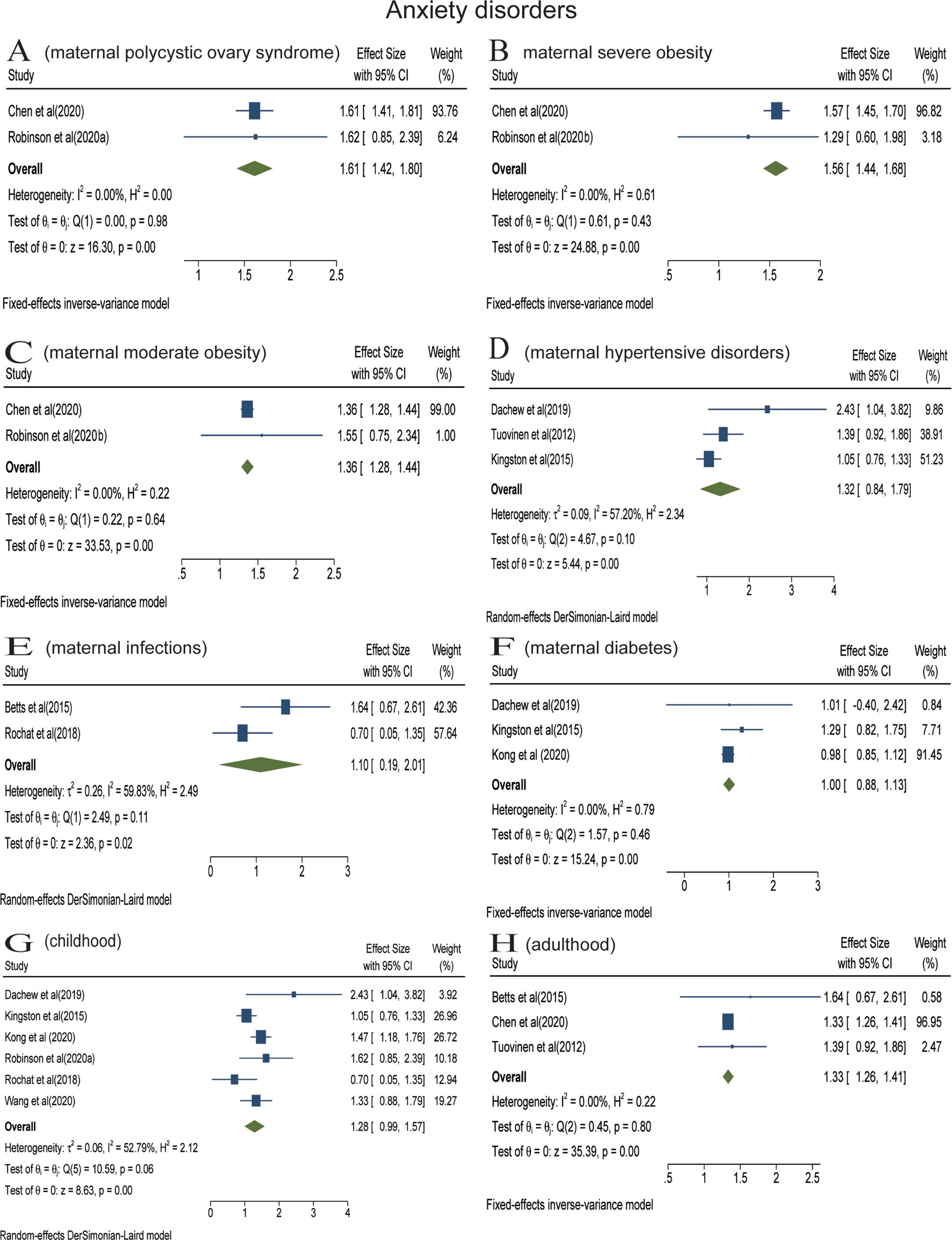
Fig. 4. Stratified analyses of anxiety disorders [Forest plot presenting combined effect estimates; ES, effect size (relative risk)]. (a) The association between maternal polycystic ovary syndrome and anxiety disorders in offspring. (b) The association between maternal severe obesity and anxiety disorders in offspring. (c) The association between maternal moderate obesity and anxiety disorders in offspring. (d) The association between maternal hypertensive disorders and anxiety disorders in offspring. (e) The association between maternal infections and anxiety disorders in offspring. (f) The association between maternal diabetes and anxiety disorders in offspring. (g) The association between maternal somatic diseases and anxiety disorders in childhood. (h) The association between maternal somatic diseases and mood disorders in adulthood.
Sensitivity analysis did not significantly alter these findings, indicating that our results were relatively stable (online Supplementary Fig. S2).
Discussion
To our knowledge, this is the first systematic review and meta-analysis assessing the risk of offspring mood and anxiety disorders in the context of prenatal maternal somatic diseases. The overall meta-analyses confirmed that prenatal maternal somatic diseases were associated with an increased risk of mood and anxiety disorders in offspring. For mood disorders, we identified that maternal obesity, hypertensive disorders, and infertility were risk factors. For anxiety disorders, risk factors were maternal PCOS and obesity. Furthermore, our study emphasised the impact of prenatal maternal somatic diseases on mood and anxiety disorders may be long-lasting.
The overarching finding of the meta-analysis showed that prenatal maternal somatic diseases were related to offspring mood/anxiety disorders. Mood and anxiety disorders are complex mental disorders, the RRs generally <2 indicated that they each modulated risk by a relatively small. Prenatal exposure to both maternal somatic diseases and psychiatric factors implicates an adverse intrauterine environment, but they might point toward differing underlying mechanisms of an increased risk of mood/anxiety disorders. Although most of the included studies (16 of 21) adjusted maternal psychiatric disorders or distress, the effect of maternal psychosocial factors cannot be excluded, as a relationship has been reported early between somatic diseases and mental health (Verhaak, Reference Verhaak1997; Harter et al., Reference Harter, Baumeister, Reuter, Jacobi, Hofler, Bengel and Wittchen2007). The results of our meta-analysis provided evidence for prenatal origins of mood and anxiety disorders. Nonetheless, some prenatal maternal somatic diseases have an indirect effect on their offspring after childbirth. This association could be mediated through children's perceived stress [e.g. cancer (Osborn, Reference Osborn2007)], household income, and socioeconomic status [e.g. diabetes (Kim et al., Reference Kim, Jeon, Han, Kim, Lee and Kim2015)] that are relevant to maternal somatic diseases.
Prenatal maternal obesity was associated with offspring mood and anxiety disorders in the results. Obesity diagnosed before pregnancy was also included because obesity is considered to be a chronic inflammatory disease (De Lorenzo et al., Reference De Lorenzo, Gratteri, Gualtieri, Cammarano, Bertucci and Di Renzo2019). Previous studies have found that maternal obesity is associated with an increased risk of neuropsychiatric disorders, basically consistent with our findings (Rivera et al., Reference Rivera, Christiansen and Sullivan2015). The findings are also supported by animal studies, which observed that maternal obesity (high-fat diet model) increased offspring anxiety and depression-like behaviours (Sullivan et al., Reference Sullivan, Grayson, Takahashi, Robertson, Maier, Bethea, Smith, Coleman and Grove2010; Gawlinska et al., Reference Gawlinska, Gawlinski, Korostynski, Borczyk, Frankowska, Piechota, Filip and Przegalinski2021). Maternal obesity is characterised by elevated systemic levels of nutrients (fatty acids, glucose), hormones (leptin, insulin) and inflammatory markers (C-reactive protein, interleukin and tumour necrosis factor) (Gil-Campos et al., Reference Gil-Campos, Canete and Gil2004; Challier et al., Reference Challier, Basu, Bintein, Minium, Hotmire, Catalano and Hauguel-de Mouzon2008; Farah et al., Reference Farah, Hogan, O'Connor, Kennelly, O'Shea and Turner2012). The pathological metabolic states and chronic inflammatory conditions influence fetal neurodevelopment (Sureshchandra et al., Reference Sureshchandra, Marshall, Wilson, Barr, Rais, Purnell, Thornburg and Messaoudi2018; Baud and Berkane, Reference Baud and Berkane2019), which may mediate the associations.
Notably, although maternal inflammation during pregnancy has been associated with offspring psychiatric disorders (Han et al., Reference Han, Patel, Jones, Nielsen, Mohammad, Hofer, Gold, Brilot, Lain, Nassar and Dale2021), we did not find that exposure to maternal infections was associated with the risk of subsequent mood and anxiety disorders. The previous review also failed to find a definitive link between maternal infections and mood disorders in offspring (Simanek and Meier, Reference Simanek and Meier2015). However, a recent meta-analysis found that maternal infection exposure was related to the risk of offspring psychosis (Zhou et al., Reference Zhou, Zhang, Chen, Hu and Jiang2021), and a nested case-control study reported a nearly fourfold increase in the risk of bipolar disorder after exposure to maternal influenza at any time during pregnancy (Parboosing et al., Reference Parboosing, Bao, Shen, Schaefer and Brown2013). The inconsistent results may suggest that the magnitude, duration and composition of inflammatory signals determine how the offspring are ultimately affected (St-Germain et al., Reference St-Germain, Castellana, Baltayeva and Beristain2020). Future studies are needed to investigate the differences of various inflammation-related diseases during pregnancy and how they affect fetal brain programming.
The results showed that hypertensive disorders during pregnancy (HDP) were associated with an increased risk of mood disorders in offspring, but the association with anxiety disorders was not statistically significant. Although a previous meta-analysis found that preeclampsia was associated with an elevated risk of offspring schizophrenia, the association with other psychiatric disorders was inconclusive (Dachew et al., Reference Dachew, Mamun, Maravilla and Alati2018). A case-control study involving 333 participants reported inconsistent results, with no significant differences in the prevalence of maternal preeclampsia between adult offspring with mood disorders and healthy controls (Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna, Luciano, Steardo, Fiorillo, Garcia and De Fazio2019). This inconsistency might be attributable to different exposure definitions, study methodology and sample sizes. The underlying mechanism of this association may be related to placental ischemia, hypoxia, inflammation and fetal programming of the hypothalamic-pituitary-adrenal axis in the context of HDP (Vitoratos et al., Reference Vitoratos, Hassiakos and Iavazzo2012; Henley et al., Reference Henley, Brown, Pennell, Lye and Torpy2016; Sharma et al., Reference Sharma, Singh, Kumar, Gupta, Rohil and Bhattacharjee2018; Socha et al., Reference Socha, Malinowski, Puk, Dubiel and Wicinski2020). In addition, HDP predicts an increased risk of preterm delivery, small for gestational age and low birth weight (Avorgbedor et al., Reference Avorgbedor, Silva, Merwin, Blumenthal and Holditch-Davis2019; Thakur and Dangal, Reference Thakur and Dangal2020; Poudel et al., Reference Poudel, Kobayashi, Miyashita, Ikeda-Araki, Tamura, Ait Bamai, Itoh, Yamazaki, Masuda, Itoh, Ito and Kishi2021), which may partly mediate the association between HDP and offspring mood disorders (Su et al., Reference Su, D'Arcy and Meng2021).
Offspring exposure to maternal PCOS was associated with an increased risk of anxiety disorders in the meta-analysis, consistent with the results of animal studies (Hu et al., Reference Hu, Richard, Maliqueo, Kokosar, Fornes, Benrick, Jansson, Ohlsson, Wu, Skibicka and Stener-Victorin2015; Risal et al., Reference Risal, Manti, Lu, Fornes, Larsson, Benrick, Deng, Cesta, Rosenqvist and Stener-Victorin2021). Elevated circulating androgen is a characteristic clinical feature of PCOS, which hypothetically could be related to an increased risk of offspring anxiety disorders (Lombardo et al., Reference Lombardo, Ashwin, Auyeung, Chakrabarti, Lai, Taylor, Hackett, Bullmore and Baron-Cohen2012; Risal et al., Reference Risal, Manti, Lu, Fornes, Larsson, Benrick, Deng, Cesta, Rosenqvist and Stener-Victorin2021). In addition, women with PCOS are more likely to seek help from assisted reproductive treatment due to common subfertility. Moreover, PCOS is associated with high rates of pregnancy complications and perinatal problems (Teede et al., Reference Teede, Misso, Costello, Dokras, Laven, Moran, Piltonen, Norman and International2018). Assisted reproductive treatment, pregnancy complications and perinatal problems are risk factors for psychosis (Davies et al., Reference Davies, Segre, Estrade, Radua, De Micheli, Provenzani, Oliver, de Pablo G, Ramella-Cravaro, Besozzi, Dazzan, Miele, Caputo, Spallarossa, Crossland, Ilyas, Spada, Politi, Murray, McGuire and Fusar-Poli2020; Rissanen et al., Reference Rissanen, Gissler, Lehti and Tiitinen2020), which may partially mediate the association.
Children born to women with infertility were at an increased risk of developing mood disorders in our study. Although the birth of offspring seems to mean that infertility has been effectively treated, preexisting pathological conditions of infertility may still affect the foetus. For example, PCOS and reproductive inflammation are common causes of female infertility, though successful pregnancy, excess androgens and inflammatory factors could persist during pregnancy and affect fetal brain development. In addition, women with infertility are more likely to have obstetric complications, premature birth and low-birth-weight infants (Thomson et al., Reference Thomson, Shanbhag, Templeton and Bhattacharya2005; Luke et al., Reference Luke, Stern, Hornstein, Kotelchuck, Diop, Cabral and Declercq2016). These predict an increased risk of mood disorders in offspring. However, we cannot identify the role of fertility treatment in the association. Medications used for fertility treatment are given before or early in pregnancy, thus the embryos may be affected by them, but there is a lack of relevant researches (Koren et al., Reference Koren, Barer and Cem Kaplan2020). It has been reported that children conceived by assisted reproductive treatment (ART) are at a higher risk of being diagnosed with autism (Liu et al., Reference Liu, Gao, He, Cai, Wang and Fan2017), mental retardation (Sandin et al., Reference Sandin, Nygren, Iliadou, Hultman and Reichenberg2013) and any other psychiatric disorders (Rissanen et al., Reference Rissanen, Gissler, Lehti and Tiitinen2020) than those naturally conceived. It is noteworthy that these control groups were composed of children naturally conceived, rather than children whose parents experienced infertility but did not use ART. Therefore, we are unable to differentiate the role that the fertility treatment played in the association from that of infertility itself. Future studies should further examine the preexisting pathology of infertility and offspring health outcomes.
The study did not find an association between maternal diabetes and anxiety disorders in offspring, which is consistent with the previous systematic reviews (Stahlberg et al., Reference Stahlberg, Khanal, Chudal, Luntamo, Kronstrom and Sourander2020). Noticeably, however, that one of the included studies showed that the association became statistically significant when maternal diabetes combined with severe obesity, and was stronger than that of either alone (Kong et al., Reference Kong, Nilsson, Brismar, Gissler and Lavebratt2020). It may reflect that the combination of diabetes and severe obesity leads to a worse intrauterine environment.
Due to the limited number of studies, maternal thyroid disease, rheumatoid arthritis, asthma, cancer, etc., were not included in the stratified analyses. The impact of these maternal somatic diseases on offspring mood and anxiety disorders deserves further investigation. For example, the association between maternal cancer prenatally diagnosed and mood disorders (HR = 2.45; 95% CI 1.02–5.89) was significantly stronger than those postnatally diagnosed (HR = 1.43; 95% CI 1.14–1.79) in one of our included studies (Momen et al., Reference Momen, Ernst, Arendt, Olsen, Li, Gissler and Ramlau-Hansen2019). However, the findings of Chen et al. suggested that parental cancer during pregnancy was not associated with the overall risk of mental illness in offspring (Chen et al., Reference Chen, Regodon Wallin, Noren Selinus, Sjolander, Fall, Valdimarsdottir, Czene and Fang2018).
In the context of maternal somatic diseases, the risk of mood disorders was increased in both childhood and adulthood, as was the risk of anxiety disorders in adulthood. The findings suggested that the effects of prenatal maternal somatic diseases were long-lasting and even persist into old age. However, the results should be interpreted with caution because the two ages were not evaluated in the same study.
There are some limitations as well. First, although a total of 21 studies were included, the majority of the studies were from northern European populations, and there was a lack of data from Asian populations. Second, when it came to specific exposures, the number of studies was small, and for some exposures, there were not enough studies to allow quantitative analysis. Third, a pooled risk consistently adjusted for the same variables could not be reached because different variables are adjusted in each study. Forth, different diagnostic criteria and follow-up periods were adopted for the same outcome, which may be the reason for the significant heterogeneity of some results. Fifth, in the context of maternal somatic disease, the effects of the disease itself and treatment measures are included, yet we cannot distinguish their effects in the associations. Sixth, it may be difficult to diagnose children, though mood and anxiety disorders occur at any time throughout the lifespan. And both mood and anxiety disorders may not be diagnosed for a long time after the onset. Therefore, the real risk may be underestimated.
In conclusion, the results of our study indicate that prenatal maternal somatic diseases can be associated with offspring mood and anxiety disorders, and that the associations may be long-lasting. These findings advance the understanding of the prenatal origins of risk for mood and anxiety disorders. More high-quality prospective studies are needed to resolve the limitations mentioned above.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796021000706
Data
Data extraction tables will be made available to any interested parties on request to the corresponding author.
Acknowledgements
We acknowledge support from the Research Center for Basic Integrative Medicine, Guangzhou University of Chinese Medicine.
Author contributions
X.G., Z.F., L.W. and C.Y. were involved in study design and protocol development. X.G., Z.F., H.W., H.X., N.Z. and L.L. led data abstraction and analysis. All authors critically reviewed and contributed to the manuscript.
Financial support
This work was supported by the National Natural Science Foundation of China (No.81774181, 81774102) and the Major Basic Research Project of Educational Commission of Guangdong Province of China (2019KZDXM022). The funding source had no influence on study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the article for publication.
Conflict of interest
None.