DSM-5 and ICD-11: business as usual?
In the light of the recent publication of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), there is renewed debate about the relative merit of categorical diagnosis, as laid down in DSM and ICD diagnostic manuals, in psychiatry. Issues such as validity, usefulness and acceptability of the diagnoses in this manual have come to the fore like never before (McGorry & van Os, Reference McGorry and van Os2013).
Expanding diagnostic categories and imbalanced inclusion of risk syndromes
The most often voiced criticisms are of a rather fundamental nature. The fact that from DSM-I to DSM-5, the number of diagnostic categories has increased from around 100 to over 300 categories, in combination with reliabilities that even in specialized field trials do not exceed kappa's of 0.6 for the majority of syndromes (Regier et al. Reference Regier, Narrow, Clarke, Kraemer, Kuramoto, Kuhl and Kupfer2013), adds to the impression of arbitrariness of categories. There was concern about the inclusion of risk syndromes in DSM-5, which many considered premature in the light of the available evidence. DSM-5 now contains a major imbalance, as a controversial risk syndrome for dementia (Mild Neurocognitive Disorder) has found its way into the manual, while another controversial risk syndrome for psychotic disorder (Attenuated Psychosis Syndrome) was discarded. Both risk syndromes faced similar inconclusive evidence regarding definition, risk function, validity, reliability and treatability, yet one was included and the other not. The main source of confusion with regard to these risk syndromes is the failure to take into account the fact that patients with early symptoms who have passed many filters along the pathway to care, and end up in specialized outpatient clinics, have much higher probabilities of transition to the full syndrome than people with the same symptoms in the general population. The risk of transition therefore has more to do with selection and referral processes resulting in risk enrichment, rather than the symptomatic syndrome per se. For this reason, the definition of Attenuated Psychosis Syndrome, conceptually imperfect as it was, stipulated that individuals had to be help seeking, excluding non-help seeking individuals in the general population with low risk of transition. However, it is unclear how the work group who included Mild Neurocognitive Disorder in DSM-5 weighed the epidemiological evidence that risk of transition from Mild Neurocognitive Disorder to dementia is low for the purpose of diagnosis, and that the risk differs as a function of setting (Mitchell & Shiri-Feshki, Reference Mitchell and Shiri-Feshki2009). Indeed, most people with mild cognitive impairment will not progress to dementia even after 10 years of follow-up (Mitchell & Shiri-Feshki, Reference Mitchell and Shiri-Feshki2009). In addition, with the advent of the dementia risk syndrome in DSM-5, many individuals and their families may feel compelled to undergo expensive diagnostic procedures only to find that there is no treatment even if risk is considered high.
The pluriform algorithm problem: categories really represent groups of sub-categories
Although DSM and ICD categories create the impression of relatively precise diagnostic categories, the fundamental structure of each category is that of a pluriform algorithm, involving a number of criteria, typically formulated as “three or more of the following symptoms…”. This type of shuffling with many different symptoms creates a range of arbitrary sub-categories within the diagnostic category, formed by all possible combinations of signs and symptoms contained with the algorithm. The result is that diagnostic categories are inherently heterogeneous, representing widely different symptom profiles, which is one of the reasons why they cannot be linked consistently to aetiology, treatment and prognosis. Given that the basic function of diagnosis is to provide information about need for care and prognosis, the pluriform algorithmic nature of DSM and ICD appears to undermine rather than facilitate clinical practice. Similarly, the practice to create widely different combinations of signs, symptoms and behaviours that nonetheless are subsumed under the same diagnostic category is unlikely to advance science, particularly if science is conducted within DSM- and ICD-like silos, as it tends to be.
Patient perspective
While many patients and their families may experience initial relief when receiving a DSM or ICD label, often this is temporary as many will face a bewildering change in diagnosis, or an expanding list of ‘comorbid’ diagnoses. In addition, over time, many will learn that the diagnosis does not refer to a natural illness type but an ever-changing convention of arbitrary criteria, which change with each revision of the diagnostic manual, and differ between DSM and ICD. Giving a person a categorical diagnosis of a ‘disorder’ that does not refer to a disease entity in nature can be considered as an act of stereotyping that may contribute to stigma and exclusion that patients experience. In addition, those receiving mystifying labels like schizophrenia will feel written off when confronted with its stereotypical standing as ‘devastating brain disease’ in academic psychiatry. Patients experience pressure to conform to the stereotype of a brain-diseased individual, resulting in self-stigma in addition to societal stigma. In this context, ‘recovery’ becomes the act of a patient constructing his own narrative and overcoming the diagnosis (Boevink, Reference Boevink2006).
Possible improvements
Cross-cutting dimensions did not make it to DSM-5
One of the possible areas of innovation that DSM-5 would introduce was the addition of cross-cutting ‘dimensions’. For example, symptoms of anxiety and depression are common in many if not most mental disorders, suggesting the use of cross-diagnostic dimensions of anxiety and depression. The addition of cross-cutting dimensions would have created a fully dimensionalized system of diagnosis, recognizing, for example, that expression of psychosis is also common – and clinically highly relevant (Perlis et al. Reference Perlis, Uher, Ostacher, Goldberg, Trivedi, Rush and Fava2011; Wigman et al. Reference Wigman, van, Vollebergh, Lieb, Beesdo-Baum, Wittchen and van Os2012, Reference Wigman, van Os, Abidi, Huibers, Roelofs, Arntz, Kelleher and Peeters2013a) – in disorders of anxiety and depression and, vice versa, that negative symptoms are also present in bipolar disorder. The addition of dimensions would have reduced the pervasive but scientifically non-productive view of psychopathology representing a myriad of categorically defined disorders, each with their own causes, diagnostic procedure and treatment. It would have opened the way to examination of affective dysregulation, aberrant salience, fear and perceptual alteration independent of the bias of specific categorical disorders. Unfortunately, it did not prove possible to develop valid methodology for cross-cutting dimensions within the time frame of DSM-5. In the short term, inclusion of cross-cutting dimensions is still a viable and important option, and may be explored in future revisions.
The Research Domain Criteria (RDoC) initiative: phenotypes that can be linked to biology but not to symptoms?
The view expressed by the NIMH is that progress in the diagnosis and treatment of mental disorders is crucially dependent on linking psychopathology to underlying biological functions. According to this view, in its essential form, genetic alterations underlie brain alterations that ‘cause’ mental disorders (Akil et al. Reference Akil, Brenner, Kandel, Kendler, King, Scolnick, Watson and Zoghbi2010). As research suggests that DSM categories cannot be linked conclusively to underlying biological dysfunctions, let alone diagnostically (Kapur et al. Reference Kapur, Phillips and Insel2012), NIMH has proposed alternative phenotypes that may be closer to the underlying biology that is considered to underlie psychopathology (Cuthbert & Insel, Reference Cuthbert and Insel2010). The RDoC project aims to study basic dimensions of functioning (such as fear, arousal, reward and working memory) to be studied across multiple units of analysis, from genes to neural circuits to behaviours, cutting across disorders as traditionally defined. While interesting, it is not clear how the chosen phenotypes relate to the symptoms that patients' experience, although the implicit suggestion is that they represent the building blocks of psychopathology. For example, it is unclear how the chosen RDoC phenotypes can be linked to psychotic symptoms such as delusions or hallucinations. Therefore, while RDoC phenotypes may represent better targets for experiments linking brain and behaviour in general, they also represent a move away from the experiences of patients, possibly decreasing their relevance for psychiatry.
Psychopathology as a symptom network
Novel analyses suggest that diagnostic categories in reality may represent complex fuzzy, cross-level causal sets that share signs and symptoms (Borsboom et al. Reference Borsboom, Cramer, Schmittmann, Epskamp and Waldorp2011; Kendler et al. Reference Kendler, Zachar and Craver2011; Kendler, Reference Kendler2012; Bringmann et al. Reference Bringmann, Vissers, Wichers, Geschwind, Kuppens, Peeters, Borsboom and Tuerlinckx2013) that develop in stages over time (McGorry & van Os, Reference McGorry and van Os2013; Wigman et al. Reference Wigman, van Os, Thiery, Derom, Collip, Jacobs and Wichers2013b). For example, stress may lead to insomnia, leading in turn to low mood, which then may cause paranoia, finally resulting in social withdrawal (Freeman et al. Reference Freeman, Stahl, McManus, Meltzer, Brugha, Wiles and Bebbington2012; de Wild-Hartmann et al. Reference de Wild-Hartmann, Wichers, van Bemmel, Derom, Thiery, Jacobs, van Os and Simons2013). Symptoms thus develop from an initial environmental disturbance, followed by a sequence of causal mental events. In this example, paranoia may in turn fuel depression, and depression insomnia, giving rise to negative feedback loops within a network of symptoms (van Os, Reference van Os2013). The importance of the network concept resides in the fact that it indicates that diagnosis should not focus only on latent constructs of categories or dimensions, but also on how symptoms impact on each other over time, giving rise to complex symptom circuits (Bringmann et al. Reference Bringmann, Vissers, Wichers, Geschwind, Kuppens, Peeters, Borsboom and Tuerlinckx2013). In other words, although clinicians naturally are used to think that the symptoms that patients display are indicators of an underlying disease, categorically or dimensionally defined, in fact there may be no strong evidence of underlying diseases, only dynamic circuits of mutually impacting symptoms (Cramer et al. Reference Cramer, Waldorp, van der Maas and Borsboom2010; Borsboom et al. Reference Borsboom, Cramer, Schmittmann, Epskamp and Waldorp2011; Kendler et al. Reference Kendler, Zachar and Craver2011). Each patient may present with a unique symptom circuit that gradually develops over time (van Os et al. Reference van Os, Delespaul, Wigman, Myin-Germeys and Wichers2013; Wigman et al. Reference Wigman, van Os, Thiery, Derom, Collip, Jacobs and Wichers2013b). Of course, network models need not be confined to mental symptoms, given strong links between mental and somatic disorders. For example, a person may develop obesity, which may lead to hypertension, which in turn may result in subclinical cardiovascular infarction, followed by arrhythmias, which subsequently cause panic attacks.
Motor dysfunction: a missing link to make diagnosis neurally informative?
In addition to the above areas of possible novel avenues for psychiatric diagnosis, it is proposed that motor dysfunctions represent a possibly underrepresented area in psychiatric diagnosis. Although ‘mental’ signs and symptoms to date have been core in the conceptualization of mental disorders, motor signs also may be relevant for the diagnostic framework, notably in the area of catatonia (Walther & Strik, Reference Walther and Strik2012). (Neurological) motor signs can be linked more directly to brain alterations. Therefore, it may be argued that motor signs should become more prominent in the psychiatric diagnostic process, facilitating examination of links between brain alterations and mental disorders. However, more work is required to relate ‘motor’ and ‘mental’ brain phenotypes in the same diagnostic framework. Basal ganglia disorders represent a natural link between movement disorders on the one hand, and behavioural and mental disorders on the other. Both are likely mediated by specific basal–ganglia–thalamocortical circuits (Graybiel, Reference Graybiel2000; Kandel, Reference Kandel2013). The association between motor dysfunction and psychopathology is well established as many movement disorders, including idiopathic dystonia and tics, are associated with increased risk of psychopathology. Furthermore, neurodegenerative disorders that are characterized by movement disorder such as Parkinson's or Huntington's disease have very high rates of psychiatric morbidity, which may be traced to the underlying neural areas that are affected.
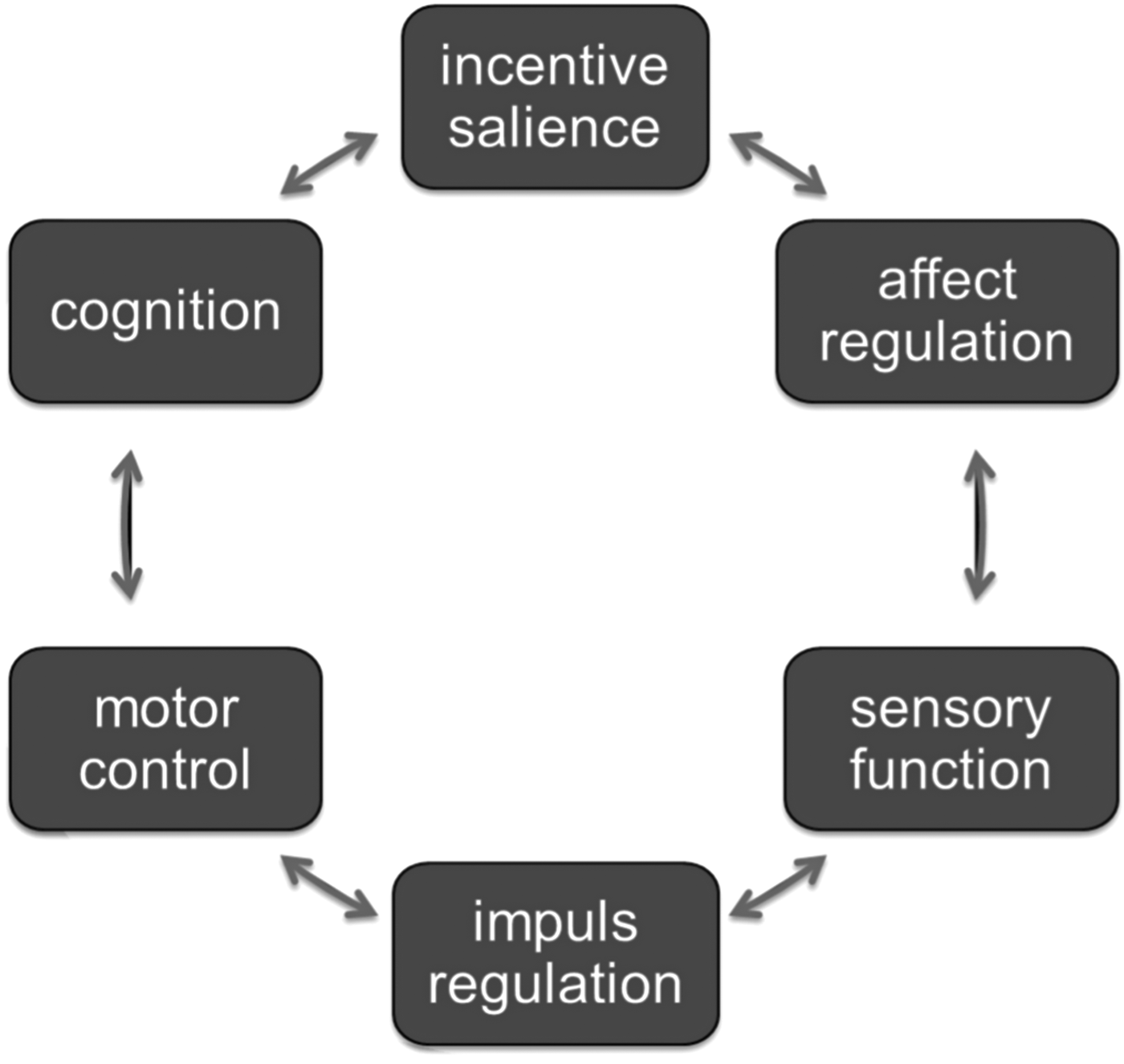
As these data point to correlations between motor dysfunction and psychiatric diagnosis, the key question is how ‘motor’ and ‘mental’ signs and symptoms may interrelate in the same mental-motor circuit, adding to the diagnostic framework. ‘Motor’ phenotypes are indicators of neural functions that regulate motor control; ‘mental’ phenotypes refer to, among others, cognition, incentive salience, affect regulation, sensory function and impulse regulation (Fig. 1). There is some evidence that all these different phenotypes interrelate in a dynamic way, possibly reflecting underlying interacting neural networks. For example, in schizophrenia, there is consistent evidence that dyskinesia is associated with measures of psychopathology (Chakos et al. Reference Chakos, Alvir, Woerner, Koreen, Geisler, Mayerhoff, Sobel, Kane, Borenstein and Lieberman1996; Murray & van Os, Reference Murray and van Os1998; Tenback et al. Reference Tenback, van Harten, Slooff and van Os2007), and akathisia may exacerbate psychopathology (Duncan et al. Reference Duncan, Adler, Stephanides, Sanfilipo and Angrist2000). A large body of literature and two meta-analyses (Pappa & Dazzan, Reference Pappa and Dazzan2009; Koning et al. Reference Koning, Tenback, van Os, Aleman, Kahn and van Harten2010) show a higher risk of dyskinesia and parkinsonism in drug-naive patients with schizophrenia, and also in first-degree relatives of patients with schizophrenia. Yarden & Discipio (Reference Yarden and Discipio1971), examining a sample of young and drug-naive patients, showed that abnormal involuntary movements were associated with an early onset and a steadily progressive course, characterized by thought disorder and poor response to medication. In addition, studies by Mittal et al. (Mittal et al. Reference Mittal, Tessner, Trottman, Esterberg, Dhrub, Simeonova, McMillan, Murphy, Saczawa and Walker2007, Reference Mittal, Neumann, Saczawa and Walker2008; Mittal & Walker, Reference Mittal and Walker2007) suggest that spontaneous motor abnormalities are markers of underlying neural alterations, forming part of schizophrenia risk. Studies indicate that orofacial dyskinesia, but not limb-truncal dyskinesia, is associated with cognitive dysfunction and negative symptoms (Waddington et al. Reference Waddington, Youssef, Dolphin and Kinsella1987; Fenton et al. Reference Fenton, Wyatt and McGlashan1994; Wolff & O'Driscoll, Reference Wolff and O'Driscoll1999). Pseudoakathisia also was associated with negative symptoms in one study (Brown & White, Reference Brown and White1991). Both tardive akathisia and tardive dyskinesia may be a marker for negative symptoms as well as for cognitive dysfunction (Sachdev et al. Reference Sachdev, Hume, Toohey and Doutney1996). A prospective study in a sample of 708 patients demonstrated that tardive dyskinesia and negative symptoms developed together (van Os et al. Reference van Os, Walsh, van Horn, Tattan, Bale and Thompson2000). Findings from another study suggest that orofacial dyskinesia and negative symptoms independently mediate spatial working memory (Pantelis et al. Reference Pantelis, Stuart, Nelson, Robbins and Barnes2001).

Fig. 1. Networked brain functions linking motor and mental functions. Different brain phenotypes interrelate in a dynamic way, giving rise to an interrelated network of experiences and functions.
Although much of the above work is correlational, and therefore not necessarily diagnostically informative, recent work indicates that motor and cognitive development may be interconnected more closely than previously thought, and that the cerebellum, striatum as well the dorsolateral prefrontal cortex may be relevant to both motor as well as cognitive performance (Diamond, Reference Diamond2000). Indeed, different studies suggest that cognitive functions are associated with drug-induced parkinsonism, for example in elderly patients (Kim et al. Reference Kim, Kim, Chung, Song, Yang, Hong, Kim, Ahn, Kim and Lee2011), as well as in patients with schizophrenia (Kim et al. Reference Kim, Kim and Byun2008; Kim & Byun, Reference Kim and Byun2009). Studies also demonstrate that (orofacial) tardive dyskinesia and cognitive dysfunctions are associated in schizophrenia (Wegner et al. Reference Wegner, Kane, Weinhold, Woerner, Kinon and Lieberman1985; Waddington & Youssef, Reference Waddington and Youssef1996; Byne et al. Reference Byne, White, Parella, Adams, Harvey and Davis1998; Dodge & Goldberg, Reference Dodge and Goldberg1999; Krabbendam et al. Reference Krabbendam, van Harten, Picus and Jolles2000), as well as in affective disorders (Wolf et al. Reference Wolf, Ryan and Mosnaim1983; Waddington & Youssef, Reference Waddington and Youssef1988). In contrast, one study showed superior cued response performance, but equal spatial memory ability, in patients with dyskinesia (Collerton et al. Reference Collerton, Fairbairn and Britton1985). In addition, findings show that (upper body) movement abnormalities are correlated with neurocognitive deficits in the prodromal period of schizophrenia (Weinberger, Reference Weinberger1996; Mittal et al. Reference Mittal, Walker, Bearden, Walder, Trottman, Daley, Simone and Cannon2010).
Although the above findings suggest that alterations of the prefrontal cortex play a possible role in tardive dyskinesia, negative symptoms and cognitive dysfunctions (Kirkpatrick & Buchanan, Reference Kirkpatrick and Buchanan1990; Levin, Reference Levin1984; Goldman-Rakic & Selemon, Reference Goldman-Rakic and Selemon1997; Byne et al. Reference Byne, White, Parella, Adams, Harvey and Davis1998), it is not clear to what degree cognitive dysfunctions (i) are a consequence or a precursor of tardive dyskinesia, (ii) represent an organic vulnerability factor or state marker for TD, or (iii) arise from the same pathophysiologic process as TD (Waddington et al. Reference Waddington, O'Callaghan, Larkin and Kinsella1993; Dodge & Goldberg, Reference Dodge and Goldberg1999).
Nevertheless, the findings (i) corroborate the hypothesis of a common abnormality of basal–ganglia–thalamocortical circuits on the one hand, and indicators of neurodevelopmental liability (cognitive dysfunction, negative symptoms) in schizophrenia and possibly other mental disorders on the other, and (ii) suggest that motor dysfunction reflects an underlying alteration of the dopamine system that also increases risk for schizophrenia. Indeed, subtle dyskinesia was associated with subthreshold psychotic experiences in the general population in a recent study (Mittal et al. Reference Mittal, Dean, Pelletier and Caligiuri2011). Although the dynamics between motor and mental phenotypes remain poorly understood, the data suggest that motor alterations beyond catatonia are diagnostically relevant, particularly as possible marker of neurodevelopmental alterations. It would be informative if the diagnostic assessment included information on neurodevelopmental liabilities that impact treatment needs and prognosis (Murray, Reference Murray1994).
Assessment technologies
It has been pointed out that collection of diagnostic information in daily life, carried out by patients, has many advantages, creating a collaborative model of a contextually relevant, personal diagnosis that is sensitive to treatment needs and prognosis (van Os et al. Reference van Os, Delespaul, Wigman, Myin-Germeys and Wichers2013). Momentary assessment techniques are available to measure mental symptoms in daily life (Csikszentmihalyi & Larson, Reference Csikszentmihalyi and Larson1987; Delespaul, Reference Delespaul1995; Myin-Germeys et al. Reference Myin-Germeys, Oorschot, Collip, Lataster, Delespaul and van Os2009), while sensor devices to assess motor function are currently being piloted. The advantage of sensor devices is that they are precise and able to measure subtle (subthreshold) motor phenotypes such as bradykinesia, tremor and dyskinesia prospectively in daily life. Therefore, in the near future, it will be possible to measure both mental and motor signs in daily life, possibly providing a diagnostic framework combining the two.
Conclusion
In conclusion, DSM and ICD categories, while providing a common language for psychiatry, can undermine rather than facilitate clinical practice, and cannot be linked diagnostically to underlying biological dysfunctions (Kapur et al. Reference Kapur, Phillips and Insel2012). Alternative phenotypes as proposed by NIMH, the RDoC phenotypes, may be closer to underlying neurobiology, but also may represent a move away from the experiences of patients, possibly decreasing their relevance for psychiatry. Several novel promising avenues exist to improve psychiatric diagnosis. First, the introduction of cross-cutting dimensions would represent a very helpful short-term improvement of the categorical framework. Second, a system of contextual precision diagnosis based on momentary assessment technology may be a valuable addition, or an alternative, to the diagnostic paradigm. Third, there is evidence that a more enhanced focus on motor dysfunction may be useful for psychiatric diagnosis, particularly as a possible indicator of underlying neurodevelopmental alterations. As motor dysfunction can also be measured with sensor technology in the flow of daily life, it may be combined with psychopathology measures assessed with momentary assessment technology, providing for a more complete diagnostic framework in the near future.
Financial Support
The project was supported in part by the European Community's Seventh Framework Programme under Grant agreement no. HEALTH-F2-2009-241909 (Project EU-GEI).
Conflict of Interest
None.