Hostility, as defined in the International Statistical Classification of Diseases and Related Health Problems (ICD) (World Health Organization, 2016), represents a clinical characteristic that implies the recurring and enduring ‘tendency to feel anger toward and a desire to inflict harm upon a person or group’ (Nakagawa et al., Reference Nakagawa, Takeuchi, Taki, Nouchi, Sekiguchi, Kotozaki, Miyauchi, Iizuka, Yokoyama, Shinada, Yamamoto, Hanawa, Araki, Hashizume, Kunitoki, Sassa and Kawashima2017). Specifically, it consists of ‘negative cognitive bias of strong disapproval toward others which implies thoughts and feelings of antagonism, resentment, and alienation’ (Spielberger et al., Reference Spielberger, Jacobs, Russell, Crane, Butcher and Spielberger1983). Hostility is associated with negative emotions during interpersonal interactions (Lemerise and Dodge, Reference Lemerise, Dodge, Lewis, Haviland-Jones and Barrett2008). Anger, which is related to hostility, can instead be described as an intense but time-limited negative emotional state of displeasure (Ehlers and Clark, Reference Ehlers and Clark2000). If anger is a state dimension, hostility is a trait leading to temperamental proneness to anger (Smith, Reference Smith, Siegman and Smith1994). Both hostility and anger are common behavioural features across non-clinical as well as clinical populations (Wang et al., Reference Wang, Wei, Li and Qiu2014; Fisher et al., Reference Fisher, Fava, Doros, Alpert, Henry, Huz and Freeman2015; Besteher et al., Reference Besteher, Squarcina, Spalthoff, Bellani, Gaser, Brambilla and Nenadić2017).
In schizophrenia, the presence of hostility has been reported during the acute phases of the disease (Raja and Azzoni, Reference Raja and Azzoni2005) and for longer time after hospital discharge (Ochoa et al., Reference Ochoa, Suarez, Novick, Arranz, Roca, Baño and Haro2013). In some cases hostility can lead to verbal or physical aggressiveness. The constructs of hostility and anger are related to that of aggressiveness, which concerns the behavioural dimension of passage to the act. It is present in a variety of mental disorders including schizophrenia, thus representing a serious and compelling issue in mental healthcare. The transition from cognition (hostility) to behaviour (aggressiveness) has been correlated with patients’ lack of compliance, poor social functioning and low quality of life (Galuppi et al., Reference Galuppi, Turola, Nanni, Mazzoni and Grassi2010; Ochoa et al., Reference Ochoa, Suarez, Novick, Arranz, Roca, Baño and Haro2013). Furthermore, both hostility and aggressiveness are related to lengthening of hospitalisation, increased costs as well as stigma (Wehring and Carpenter, Reference Wehring and Carpenter2011). In the current review, we aim to clarify the neural basis of hostility and related dimensions in schizophrenia.
Hostility-related behaviours observed in schizophrenia are rooted in complex and multi-factorial causes. In particular, aggressiveness has been linked to both impulsivity and psychotic symptoms (Stahl, Reference Stahl2014; Hoptman, Reference Hoptman2015). Impulsivity is a multidimensional construct defined as ‘a predisposition toward rapid unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to themselves or others’ (Moeller et al., Reference Moeller, Barratt, Dougherty, Schmitz and Swann2001). It accounts for a substantial proportion of aggressive behaviours in schizophrenia, especially in inpatient settings, and it has been associated with increased suicidal risk in this population (Ouzir, Reference Ouzir2013). Impulsive aggressiveness in schizophrenia is situationally bound, reactive and characterised by a lack of clear intent (Leclerc et al., Reference Leclerc, Regenbogen, Hamilton and Habel2018). Impulsivity is related to urgency, representing impulsivity in the context of both positive and negative strong emotions. Also, urgency is elevated in schizophrenia due to affective dysregulation (Hoptman et al., Reference Hoptman, Antonius, Mauro, Parker and Javitt2014). As for positive symptoms, paranoid delusions, hallucinations and grandiosity may lead to misinterpretation of environmental stimuli and thus episodes of irritability or aggressiveness (Stahl, Reference Stahl2014). The comorbid use of substance represents an additional risk factor for schizophrenia patients to become aggressive (Fazel et al., Reference Fazel, Langstrom, Hjern, Grann and Lichtenstein2009; Schiffer et al., Reference Schiffer, Müller, Scherbaum, Forsting, Wiltfang, Leygraf and Gizewski2010). Cognitive deficits have also been implicated in the genesis and maintenance of aggressiveness in schizophrenia through inefficient regulation of negative affective states, with global cognitive impairment, deficits in working memory, and reasoning/problem solving as the most involved (Reinharth et al., Reference Reinharth, Reynolds, Dill and Serper2014; Ahmed et al., Reference Ahmed, Richardson, Buckner, Romanoff, Feder, Oragunye, Ilnicki, Bhat, Hoptman and Lindenmayer2018). Finally, social emotion processing deficits such as impaired facial affect recognition have been associated with an increased risk of aggressiveness in psychosis (Malone et al., Reference Malone, Carroll and Murphy2012). A recent study by Krakowski and Czobor (Reference Krakowski and Czobor2017) pointed at the existence of two distinct profiles of proneness to aggressiveness in schizophrenia combining personality traits, cognitive function and emotional processes: the first patient group was characterised by impulsivity, psychopathy, deficits in cognition and fear recognition, with proneness to aggressiveness. The second profile was defined by impairment in facial affect processing and cognitive perseveration, and has an inverse relationship with aggression. Another study by Bilgi et al. (Reference Bilgi, Taspinar, Aksoy, Oguz, Coburn and Gonul2017) suggested a relationship among misidentification of emotional faces and patients’ irritability, which was in turn related to childhood adversity experienced by some patients with schizophrenia. Interestingly, cognitive remediation and social cognitive training have been recently suggested to complement the action of the traditional (mainly pharmacological) care tools in the management of hostility and aggressiveness in schizophrenia (Darmedru et al., Reference Darmedru, Demily and Franck2018).
The measurement of hostility, impulsiveness and aggressiveness can be critical for patient management and treatment planning. A series of psychometric instruments have been created to assess such dimensions. In particular, the Buss Durkee Hostility Inventory (BDHI) (Buss and Durkee, Reference Buss and Durkee1957) is a 75-item self-report inventory comprising height subscales measuring multidimensional aspects of anger/hostility and aggression (i.e. negativism, indirect hostility and irritability). The revision of the BDHI item pool has resulted in the emergence of the Buss-Perry Aggression Questionnaire (BPAQ) (Buss and Perry, Reference Buss and Perry1992) which includes 29 items covering physical aggression, verbal aggression, anger and hostility. Other scales are the Novaco Anger Scale (NAS) (Novaco, Reference Novaco2003), which comprises 25 hypothetical situations that are likely to provoke anger, and the Multidimensional Anger Inventory (MAI) (Siegel, Reference Siegel1986) measuring multiple dimensions of anger, including frequency, duration, magnitude and mode of expression (anger-in or anger-out). The Barratt Impulsiveness Scale (BIS) (Patton et al., Reference Patton, Stanford and Barratt1995) is one of the most common self-report measures of impulsivity. Also instruments routinely used in clinical practice and research like the Positive and Negative Syndrome Scale (PANSS) and the Symptom Checklist 90 (SCL-90) (Derogatis, Reference Derogatis1983) include subscales measuring hostility and related behaviour. In particular, the PANSS subscale ‘Hostility’ is part of the positive scale and measures verbal and non-verbal expressions of anger and resentment, including sarcasm, passive-aggressive behaviour, verbal abuse and physical aggressiveness. The SCL-90 hostility subscale is made up of six items assessing thoughts, feelings and actions related to resentment, irritability, aggression and rage. It has to be specified that different scales measure different shades of the cited constructs therefore the choice of the best scale depends on the specific aspects that clinicians or researchers are interested to measure (see Fernandez et al., Reference Fernandez, Day, Boyle, Boyle, Saklofske and Matthews2014 for a detailed review on measures of anger/hostility/aggression in adults).
An extensive understanding of the neurobiological patterns involved in hostility, impulsivity and aggressiveness in schizophrenia is crucial to comprehend the pathophysiology of these aspects, which directly affect the clinical management of patients. In the current paper, we reviewed the neuroimaging literature on hostility and related dimensions in schizophrenia. We focused on structural magnetic resonance imaging (sMRI) techniques with some findings from functional magnetic resonance imaging (fMRI) studies. A bibliographic search on PUBMED was performed and the search terms were ‘hostility’, ‘impulsivity’, ‘urgency’, ‘aggressiveness’, ‘violence’, ‘schizophrenia’ and ‘magnetic resonance imaging’. Aggressive behaviour and impulsivity are the most investigated hostility-related dimensions in neuroimaging investigations in schizophrenia. Hoptman et al. (Reference Hoptman, Volavka, Johnson, Weiss, Bilder and Lim2002) found an association between disrupted white matter in the right inferior frontal area and high level of both impulsiveness and aggressiveness in a group of 14 men with schizophrenia who had shown aggressive behaviour. Other studies reported that higher scores of aggression correlated with larger grey and white matter volumes in the caudate as well as larger left orbitofrontal (OFC) grey matter and bilateral OFC white matter volumes on treatment-resistant patients with schizophrenia (Hoptman et al., Reference Hoptman, Volavka, Weiss, Czobor, Szeszko, Gerig, Chakos, Blocher, Citrome, Lindenmayer, Sheitman, Lieberman and Bilder2005, Reference Hoptman, Volavka, Czobor, Gerig, Chakos, Blocher, Citrome, Sheitman, Lindenmayer, Lieberman and Bilder2006). A more recent study from the same group found a significant reduction in functional connectivity between amygdala and ventral prefrontal cortex (vPFC) regions, where fractional anisotropy (FA) along connecting tracts was inversely related to aggression measured with the BPAQ in patients with schizophrenia (Hoptman et al., Reference Hoptman, D'Angelo, Catalano, Mauro, Shehzad, Kelly, Castellanos, Javitt and Milham2010). A recent systematic review and effect size analysis of structural MRI studies by Widmayer et al. (Reference Widmayer, Sowislo, Jungfer, Borgwardt, Lang, Stieglitz and Huber2018) showed lower total as well as regional prefronto-temporal, hippocampus, thalamus and cerebellum brain volumes and higher volumes of lateral ventricles, amygdala and putamen in aggressive v. non-aggressive people with schizophrenia. Impulsivity, on the other hand, has been mostly investigated with fMRI. A comprehensive review of both structural and functional MRI studies in schizophrenia was performed by Ouzir (Reference Ouzir2013), showing a relationship between impulsivity and activation deficits in dorso-/ventro-lateral prefrontal cortex (DLPFC, VLPFC) and anterior cingulate cortex (ACC). In contrast, a study from Nanda et al. (Reference Nanda, Tandon, Mathew, Padmanabhan, Clementz, Pearlson, Sweeney, Tamminga and Keshavan2016) did not show any correlation between BIS impulsivity scores and brain measures in schizophrenia. Neural correlates of urgency have been less intensely studied in schizophrenia v. healthy controls. In particular, Hoptman et al. (Reference Hoptman, Antonius, Mauro, Parker and Javitt2014) combining an automated MRI and resting state methods showed increased urgency predicting lower cortical thickness in ventral-prefrontal areas, as well as lower connectivity between this region and both limbic and executive brain regions beyond the diagnostic classification. In the same study, urgency and aggressive attitudes were significantly elevated in patients with schizophrenia compared with healthy controls, showing an associated reduced resting-state functional connectivity in the same brain regions only in patients. Overall, studies on schizophrenia showed abnormally higher levels of urgency, impulsivity and aggressiveness. Interestingly, studies comparing aggressive v. non-aggressive subjects with schizophrenia suggest different anatomical patterns of altered volumes and connectivity in the two groups, supporting the existence of different profiles of proneness to aggressiveness and related neuroanatomical alterations in this population (Kumari et al., Reference Kumari, Das, Taylor, Barkataki, Andrew, Sumich, Williams and Ffytche2009, Reference Kumari, Uddin, Premkumar, Young, Gudjonsson, Raghuvanshi, Barkataki, Sumich, Taylor and Das2014; Schiffer et al., Reference Schiffer, Leygraf, Müller, Scherbaum, Forsting, Wiltfang, Gizewski and Hodgins2013; Krakowski and Czobor, Reference Krakowski and Czobor2017; Kuroki et al., Reference Kuroki, Kashiwagi, Ota, Ishikawa, Kunugi, Sato, Hirabayashi and Ota2017; Widmayer et al., Reference Widmayer, Sowislo, Jungfer, Borgwardt, Lang, Stieglitz and Huber2018). In Table 1, we summarise the structural brain imaging studies on the anatomical substrates of hostility-related dimensions in schizophrenia.
Table 1. Structural MRI studies on the neuroanatomical correlates of hostility-related dimensions in schizophrenia
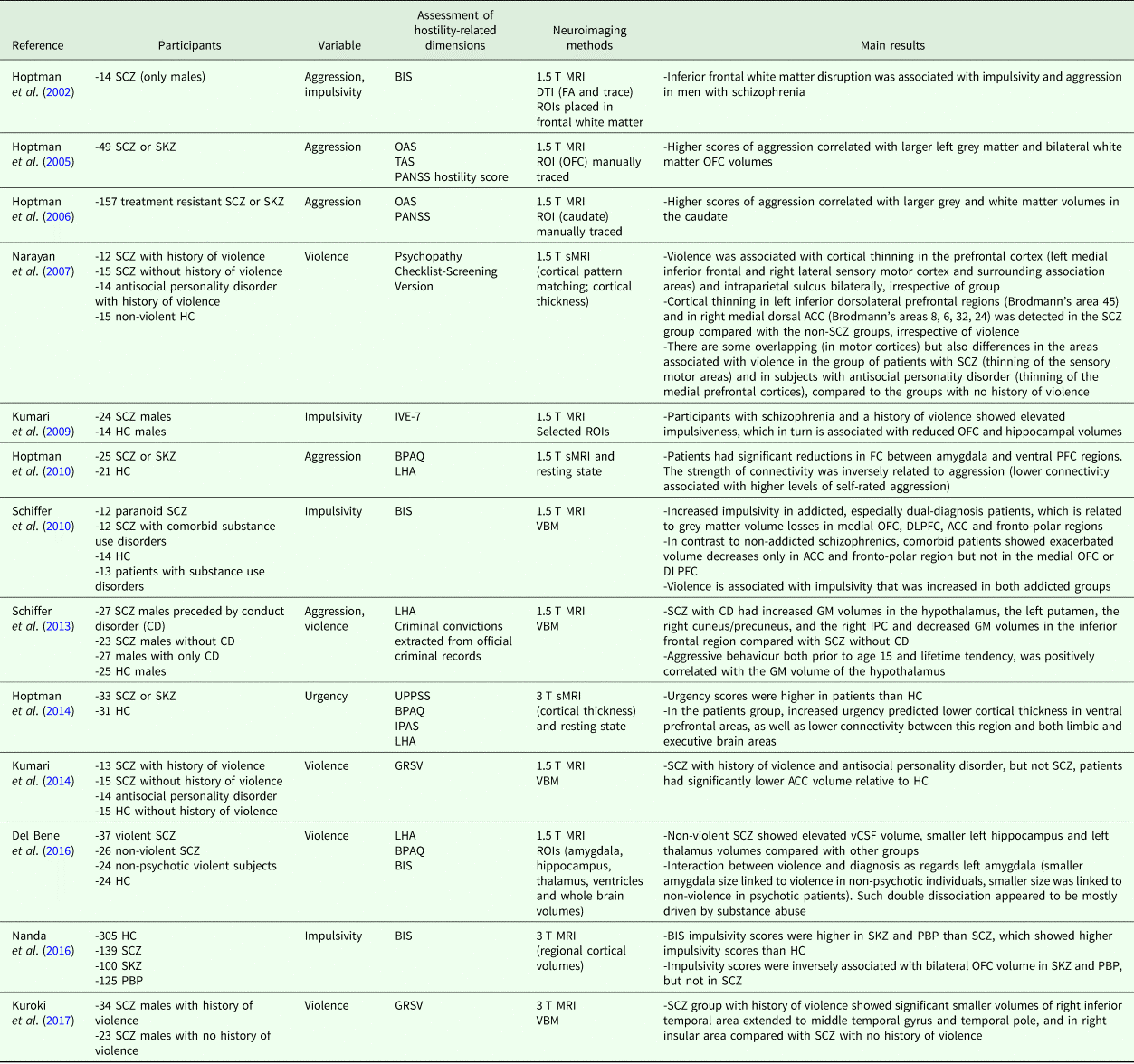
ACC, anterior cingulate cortex; BD, patients with bipolar disorder; BIS, Barratt Impulsiveness Scale; BPAQ, Buss-Perry Aggression Questionnaire; CD, conduct disorder; vCSF, ventricular cerebrospinal fluid; DLPFC, dorso-lateral prefrontal cortex; DTI, diffusion tensor imaging; GM, grey matter; GRSV, Gunn and Robertson Scale for Violence (Gunn and Robertson, Reference Gunn and Robertson1976); HC, healthy controls; IPAS, Impulsive and Premeditated Aggression Scale (Stanford et al., Reference Stanford, Houston, Mathias, Villemarette-Pittman, Helfritz and Conklin2003); IPC, inferior parietal cortex; IVE-7, Impulsiveness–Venturesomeness–Empathy questionnaire (Eysenck et al., Reference Eysenck, Pearson, Easting and Allsopp1985); LHA, life history of aggression; OAS, Overt Aggression Scale; OFC, orbito-frontal cortex; PANSS, Positive and Negative Symptoms Scale; PBP, patients with psychotic bipolar disorder; PFC, prefrontal cortex; ROI, region of interest; SCZ, patients with schizophrenia; SKZ, patients with schizoaffective disorders; sMRI, structural magnetic resonance imaging; T, tesla; TAS, Total Aggression Severity score; UPPSS, Urgency, Premeditation, Perseverance and Sensation Seeking scale (Cyders and Smith, Reference Cyders and Smith2007); VBM, voxel-based morphometry.
Neuroanatomical observations made in people with schizophrenia are in line with results from non-clinical samples (Besteher et al., Reference Besteher, Squarcina, Spalthoff, Bellani, Gaser, Brambilla and Nenadić2017; Nakagawa et al., Reference Nakagawa, Takeuchi, Taki, Nouchi, Sekiguchi, Kotozaki, Miyauchi, Iizuka, Yokoyama, Shinada, Yamamoto, Hanawa, Araki, Hashizume, Kunitoki, Sassa and Kawashima2017). Specifically, Nakagawa et al. (Reference Nakagawa, Takeuchi, Taki, Nouchi, Sekiguchi, Kotozaki, Miyauchi, Iizuka, Yokoyama, Shinada, Yamamoto, Hanawa, Araki, Hashizume, Kunitoki, Sassa and Kawashima2017) explored the brain structures underlying hostility in a large sample of young healthy subjects showing a positive correlation between hostility scores and regional grey matter density in the anterior midcingulate cortex (aMCC), which has been hence suggested as a specific neural node accounting for the cognitive aspect of hostility. This result is in accordance with the role assigned to aMCC as integrating emotional and motor information concerning others’ intentional behaviour in order to execute goal-directed behaviour (Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011). In the study of Nakagawa et al. (Reference Nakagawa, Takeuchi, Taki, Nouchi, Sekiguchi, Kotozaki, Miyauchi, Iizuka, Yokoyama, Shinada, Yamamoto, Hanawa, Araki, Hashizume, Kunitoki, Sassa and Kawashima2017), hostility was also positively associated with regional grey matter density of several frontal regions such as DLPFC and the lateral premotor cortex. More recently, a voxel-based morphometry study from our extended group (Besteher et al., Reference Besteher, Squarcina, Spalthoff, Bellani, Gaser, Brambilla and Nenadić2017) showed a positive correlation of irritability/hostility measured with SCL-90 and grey matter volume in bilateral anterior cingulate, OFC, gyrus rectus, left lingual and postcentral gyri.
Taken together, studies on patients with schizophrenia and in healthy adults with varying levels of hostility/aggressiveness suggest the existence of a broad phenotype covering manifestations of anger, hostility and aggression across clinical and non-clinical populations. A neural network including fronto-limbic and subcortical regions would underpin such a phenotype. A neuroanatomical model of impulsivity and aggressiveness has recently been proposed (Coccaro et al., Reference Coccaro, Sripada, Yanowitch and Phan2011) pointing at the existence of a continuity of hostility-related dimensions ranging from healthy subjects to pathological conditions rather than a specificity of such phenomena for schizophrenia (Narayan et al., Reference Narayan, Narr, Kumari, Woods, Thompson, Toga and Sharma2007; Leclerc et al., Reference Leclerc, Regenbogen, Hamilton and Habel2018). Such model includes brain regions involved in the generation of aggressive responses (brain stem, amygdala and striatum), emotion regulation and impulse control (DLPFC, VLPFC, dorsal ACC and OFC), decision making and socio-emotional information processing (insula, VLPFC, rostral ACC, OFC, dorso-medial prefrontal cortex-PFC and hippocampus). Brain regions included in the model were observed to be altered not only in schizophrenia but also in other clinical groups with increased levels of aggressiveness such as people with bipolar disorder, antisocial personality or conduct disorder (Leclerc et al., Reference Leclerc, Regenbogen, Hamilton and Habel2018). Some of these areas (especially amygdala, OFC, ACC and hippocampus) appear to be reduced in adult healthy controls who experienced childhood adversities (Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd, Domschke, Hohoff, Ohrmann, Bauer, Lindner, Postert, Konrad, Arolt, Heindel, Suslow and Kugel2012, Reference Dannlowski, Kugel, Huber, Stuhrmann, Redlich, Grotegerd, Dohm, Sehlmeyer, Konrad, Baune, Arolt, Heindel, Zwitserlood and Suslow2013; Teicher and Samson, Reference Teicher and Samson2013), suggesting that early mistreatment experiences can represent a shared risk factor for emotion dysregulation and distress in adult life.
Overall, neuroanatomical investigations of hostility and related dimensions are useful to map target brain areas relevant for both pharmacological and non-pharmacological (i.e. neurostimulation and cognitive remediation) treatments of hostile thoughts and behaviours in schizophrenia. Moreover, further studies comparing clinical groups with emotional dysregulation and healthy controls with and without the presence of hostility and aggressiveness are warranted to deliver more insight into our understanding of hostility-related dimensions in schizophrenia and their neuroanatomical correlates within the continuum from non-clinical conditions to pathology.
Acknowledgements
The authors thank Dr Andrea Bertolazzi for his initial input on discussing hostility in schizophrenia. P.B. was partially supported by grants from the Ministry of Health (RF-2011-02352308).
Financial support
None.
Conflict of interest
None.



