Generalised anxiety disorder (GAD) is a common psychiatric disease characterised by specific physical and psychological symptoms, including persisting worry, irritability and fatigue (DSM5, American Psychiatry Association, 2013; Diwadkar et al. Reference Diwadkar, Re, Cecchetto, Garzitto, Piccin, Bonivento, Maieron, D'Agostini, Balestrieri and Brambilla2017). GAD causes high human suffering, which is poorly understood. With respect to neuroimaging studies, the exploration of putative biomarkers of this disease is still at an early stage (Terlevic et al. Reference Terlevic, Isola, Ragogna, Meduri, Canalaz, Perini, Rambaldelli, Travan, Crivellato, Tognin, Como, Zuiani, Bazzocchi, Balestrieri and Brambilla2012). This is true especially for the application of proton Magnetic Resonance Spectroscopy (¹H MRS), which has the unique ability of providing important quantitative biochemical information in localised brain areas (Stanley, Reference Stanley2002). This can lead to identifying possible and more effective pharmacological treatments for GAD. The prominent 1H metabolites include N-acetyl-aspartate (NAA), a marker for neuronal density and functioning, glycerophosphocholine plus phosphocholine (GPC + PC), membrane phospholipid metabolites, and phosphocreatine and creatine (PCr + Cr), involved in energetic processes (Brambilla et al. Reference Brambilla, Stanley, Nicoletti, Harenski, Wells, Mallinger, Keshavan and Soares2002, Reference Brambilla, Como, Isola, Taboga, Zuliani, Goljevscek, Ragogna, Brondani, Baiano, Perini, Ferro, Bazzocchi, Zuiani and Balestrieri2012; Stanley et al. Reference Stanley, Vemulapalli, Nutche, Montrose, Sweeney, Pettegrew, MacMaster and Keshavan2007).
In this review, we aimed at providing an overview of ¹H MRS studies carried out in GAD with the final goal of shedding light on the significance of the reported altered metabolite levels in this disorder. A bibliographic search on PUBMED on ¹H MRS studies in GAD was performed and the research terms used were ‘MRS’, ‘spectroscopy’, and ‘generalised anxiety disorder’. Studies were excluded if the publications: (a) included twin samples, (b) investigated GAD not in relation to healthy controls (HC) or (c) explored only white matter structures. In total, eleven papers met the inclusion criteria and are summarised in Table 1. Briefly, among the 11 studies retrieved, the majority employed a multi-voxel (N = 7) instead of a single-voxel (N = 4) ¹H MRS technique and a 1.5 T scanner (N = 6) instead of a 3 T (N = 4) or a 4 T (N = 1) scanner. Interestingly, all the ¹H MRS studies on GAD, except for two studies Abdallah et al. Reference Abdallah, Coplan, Jackowski, Sato, Mao, Shungu and Mathew2012a ; Strawn et al. Reference Strawn, Chu, Whitsel, Weber, Norris, Adler, Eliassen, Phan, Strakowski and DelBello2013), focused on brain regions within the hippocampus and dorsolateral prefrontal cortex (DLPFC).
Table 1. Selection of studies on generalised anxiety disorder exploring metabolic alterations with 1-H magnetic resonance spectroscopy
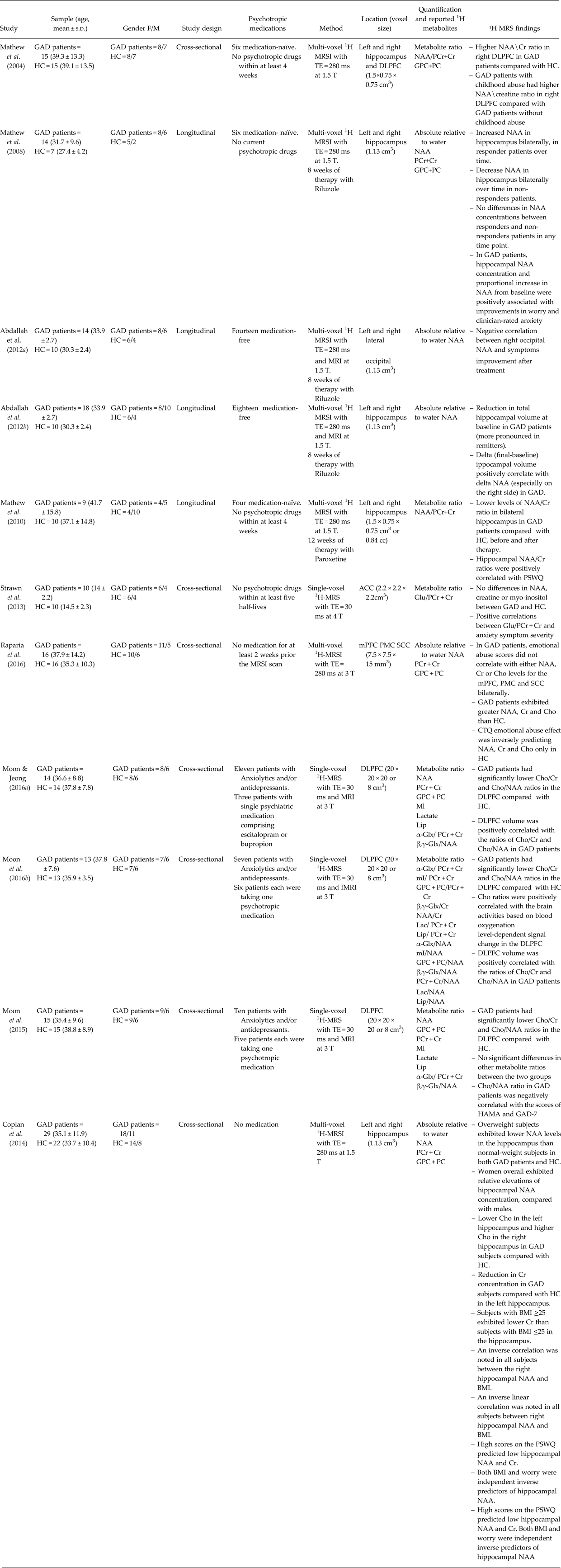
GAD, Generalised anxiety disorder; MRI, Magnetic Resonance Imaging; fMRI, Functional Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; MRSI, Magnetic Resonance Spectroscopy Imaging; NAA, N-Acetyl-Aspartate; GPC + PC, Glycerophosphocholine plus Phosphocholine; PCr + Cr, Phosphocreatine plus Creatine; HC, Healthy controls; DLPFC, Dorsolateral prefrontal cortex; ACC, Anterior Cingulate Cortex; SSC, Somatosensory cortex; mPFC, medial prefrontal cortex; BMI, Body mass index; PSWQ, Penn State Worry Questionnaire.
Regarding the DLPFC, four studies of which three of them are from the same group, reported multiple contrasts including higher ratios of NAA/PCr + Cr (Mathew et al. Reference Mathew, Mao, Coplan, Smith, Sackeim, Gorman and Shungu2004) and lower ratios of GPC + PC/PCr + Cr and GPC + PC/NAA (Moon et al. Reference Moon, Kang and Jeong2015; Reference Moon, Sundaram, Choi and Jeong2016b ; Moon & Jeong, Reference Moon and Jeong2016a ) ratios in GAD patients compared with HC. Additionally, Raparia et al. (Reference Raparia, Coplan, Abdallah, Hof, Mao, Mathew and Shungu2016) found higher NAA, PCr + Cr and GPC + PC levels in DLPFC, premotor cortex (PC) and secondary somatosensory cortex (SSC) bilaterally in GAD patients compared with HC. Interestingly, Mathew et al. (Reference Mathew, Mao, Coplan, Smith, Sackeim, Gorman and Shungu2004) reported that GAD patients with childhood abuse had higher NAA/PCr + Cr ratios compared with GAD patients without childhood abuse. In contrast, Raparia et al. (Reference Raparia, Coplan, Abdallah, Hof, Mao, Mathew and Shungu2016) found that GAD patients had no significant associations between emotional abuse scores and NAA, PCr + Cr and GPC + PC levels in DLPFC, PC and SSC bilaterally, but were significant in HC. Additionally, the three studies carried out by Moon et al. found that GPC + PC/PCr + Cr and GPC + PC/NAA ratios positively correlated with right DLPFC volumes (Moon & Jeong, Reference Moon and Jeong2016a ; Moon et al. Reference Moon, Sundaram, Choi and Jeong2016b ) and blood oxygenation level-dependent signal change in right DLPFC (Moon et al. Reference Moon, Sundaram, Choi and Jeong2016b ). In contrast, a negative correlation was observed between GPC + PC/NAA ratio and anxiety symptom severity (Moon et al. Reference Moon, Kang and Jeong2015). Collectively, these studies suggest DLPFC neuronal deficits, which may in turn explain the neurocognitive deficits often observed in GAD patients. Indeed DLPFC is a key brain area regulating cognition and emotion, and plays a prominent role in working memory and executive brain functions (Brambilla et al. Reference Brambilla, Glahn, Balestrieri and Soares2005).
Regarding the hippocampus, the study by Mathew et al. (Reference Mathew, Price, Mao, Smith, Coplan, Charney and Shungu2008) showed increased hippocampal NAA levels after 8 weeks of treatment with the glutamate-antagonist riluzole in GAD responder patients, whereas hippocampal NAA levels decreased over time in non-responders. Moreover, the change over time (post-minus pre treatment) in hippocampal volume was positively associated with change over time in NAA (especially in the right side) and with the improvement in anxiety symptoms (Abdallah et al. 2013). In contrast, lower ratios of NAA/PCr + Cr in bilateral hippocampus of nine GAD patients were not reversed after 12 weeks of paroxetine, despite marked symptoms improvement (Mathew et al. Reference Mathew, Price, Shungu, Mao, Smith, Amiel and Coplan2010). Additionally, Abdallah et al. (Reference Abdallah, Coplan, Jackowski, Sato, Mao, Shungu and Mathew2012a ) observed a negative correlation between right occipital NAA and symptoms improvement after riluzole treatment. Riluzole has been demonstrated to modulate extracellular glutamate through glial reuptake mechanisms regulating neural plasticity in the hippocampus (Frizzo et al. Reference Frizzo, Dall'Onder, Dalcin and Souza2004). SSRIs have also been linked to enhance neural plasticity in hippocampal cells (Wang et al. Reference Wang, David, Monckton, Battaglia and Hen2008). Therefore, hippocampal NAA may reflect non-neuronal activity (Mathew et al. Reference Mathew, Price, Mao, Smith, Coplan, Charney and Shungu2008) being a possible biomarker of GAD, and NAA change might be differently related to disparate mechanisms of drug action. Additionally, Coplan et al. (Reference Coplan, Fathy, Abdallah, Ragab, Kral, Mao, Shungu and Mathew2014) also reported significant metabolites alterations associated with weight, with overweight GAD patients showing lower NAA in hippocampus compared with HC. Moreover, an inverse correlation was observed between hippocampal NAA and body mass index as well as higher worry predicted low hippocampal NAA and PCr + Cr. Lastly, Strawn et al. (Reference Strawn, Chu, Whitsel, Weber, Norris, Adler, Eliassen, Phan, Strakowski and DelBello2013) recently reported no significant alterations in glutamate/PCr + Cr ratios in the anterior cingulate of adolescents with GAD; however, a positive correlations between glutamate/PCr + Cr and anxiety symptoms severity.
In conclusions, these findings together suggest that GAD is associated with metabolic dysfunctions in selective brain regions, including the DLPFC and hippocampus. However, these results require further independent replications. Indeed, although the majority of the studies employed absolute metabolite values, some others used metabolite ratios, which might have therefore limited the interpretations of the results. Additionally, most of the studies were characterised by relatively small sample sizes and were carried out by the same research group, further decreasing the generalisability of their findings. Despite these limitations, these findings illustrate that alterations in specific metabolites, especially NAA, PCr + Cr and GPC + PC, might be considered putative biomarkers of GAD. Additionally, from the ¹H MRS studies here described emerged that pharmacological treatments positively interact with specific metabolites, especially NAA, within selective brain regions. Therefore, the investigation of brain metabolites could be very effective not only for elucidating the pathophysiology of neuropsychiatric diseases, but also for the identification of more beneficial and targeted pharmacological interventions. Finally, although ¹H MRS has been combined with other neuroimaging methods in recent studies (Abdallah et al. Reference Abdallah, Coplan, Jackowski, Sato, Mao, Shungu and Mathew2012a , Reference Abdallah, Coplan, Jackowski, Sato, Mao, Shungu and Mathew b , 2013; Moon et al. Reference Moon, Kang and Jeong2015, Reference Moon, Sundaram, Choi and Jeong2016b ; Moon & Jeong, Reference Moon and Jeong2016a ), the evidence are still scarce. However, it is important to point out that the combination of more MRI methods allows the integration of different measures, which might increase the information and consequently the reliability of the findings.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.



