The lack of reliable, specific brain biomarkers for autism spectrum disorders (ASD) results in a diagnosis based on behavioural criteria (Muratori et al. Reference Muratori, Narzisi, Tancredi, Cosenza, Calugi, Saviozzi, Santocchi and Calderoni2011). However, recent structural magnetic resonance imaging (sMRI) studies provide new insights into the neuroanatomical substrate of ASD, suggesting the involvement of the corpus callosum and the fronto-parieto-temporal regions (Mengotti et al. Reference Mengotti, D'Agostini, Terlevic, De Colle, Biasizzo, Londero, Ferro, Rambaldelli, Balestrieri, Zanini, Fabbro, Molteni and Brambilla2011; Bellani et al. Reference Bellani, Calderoni, Muratori and Brambilla2013). Among these latter, the amygdala is a relatively small subcortical brain region located in the anteromedial temporal lobe and included in the limbic system. It contains at least 13 distinct nuclei, among which four major nuclei (the lateral, basal, accessory basal and central nuclei) with unique patterns of connectivity with other brain regions. In particular, the central nucleus, a phylogenically primitive part, communicates mostly with brainstem and olfactory centres, while the basolateral nuclei are strongly connected to the neocortex. Besides its primary role of monitoring the environment for potential danger and modulating levels of vigilance, the amygdala plays a seminal contribution to social behaviour. Specifically, it is implicated in several cognitive functions, including social cognition, recognition of emotions, attribution of emotional valence to stimuli and regulation of the personal space. These findings have led researchers to postulate the ‘amygdala theory of autism’ since the amygdala may be primarily involved in the socio-emotional impairment peculiar of ASD subjects (Baron-Cohen et al. Reference Baron-Cohen, Ring, Bullmore, Wheelwright, Ashwin and Williams2000).
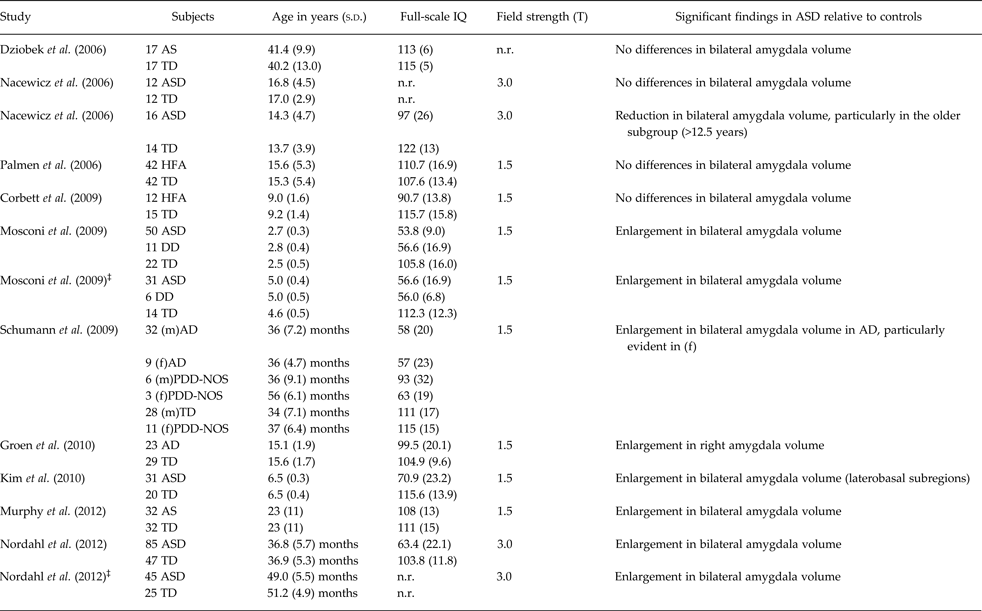
However, the presence of amygdala structural abnormalities in ASD is unclear since previous research has produced conflicting results. Indeed, increased, decreased and preserved volumes have been shown in studies using manual tracing to define the amygdala morphology (8, 1 and 4 studies, respectively; see Table 1). Nonetheless, there is some evidence for age-related effects on amygdala volumes, confirmed by a recent meta-analysis of sMRI studies in ASD (Stanfield et al. Reference Stanfield, McIntosh, Spencer, Philip, Gaur and Lawrie2008). Specifically, ASD toddlers and children frequently show significantly increased bilateral amygdala volumes relative to age-matched controls (Mosconi et al. Reference Mosconi, Cody-Hazlett, Poe, Gerig, Gimpel-Smith and Piven2009; Schumann et al. Reference Schumann, Barnes, Lord and Courchesne2009; Kim et al. Reference Kim, Lyoo, Estes, Renshaw, Shaw, Friedman, Kim, Yoon, Hwang and Dager2010; Nordahl et al. Reference Nordahl, Scholz, Yang, Buonocore, Simon, Rogers and Amaral2012), whereas older adolescents and adults either reduced (Nacewicz et al. Reference Nacewicz, Dalton, Johnstone, Long, McAuliff, Oakes, Alexander and Davidson2006), or preserved size (Corbett et al. Reference Corbett, Carmean, Ravizza, Wendelken, Henry, Carter and Rivera2009; Dziobek et al. Reference Dziobek, Fleck, Rogers, Wolf and Convit2006; Nacewicz et al. Reference Nacewicz, Dalton, Johnstone, Long, McAuliff, Oakes, Alexander and Davidson2006; Palmen et al. Reference Palmen, Durston, Nederveen and Van Engeland2006). Despite the age of the subject population seems to be a critical factor, some heterogeneity in the rate of amygdala growth within the ASD population of the same age-range has been detected. Accordingly, a recent longitudinal study pointed to three ASD subgroups in the amygdala developmental time course between two and four years of age, i.e. (1) rapid growth, (2) slow growth, and (3) growth trajectories consistent with those of typically developing children (Nordahl et al. Reference Nordahl, Scholz, Yang, Buonocore, Simon, Rogers and Amaral2012). The behavioural correlates of different amygdala growth patterns, unfortunately, are not reported in this study. In contrast, very few papers performed a separate analysis by sex, showing more pronounced amygdala enlargement in female children with ASD (Schumann et al. Reference Schumann, Barnes, Lord and Courchesne2009) compared with age- and gender-matched typically developing controls. These preliminary findings suggest a potential different pattern of amygdala development in ASD in accordance to gender.
Table 1. Summary of studies published between 2006–2012 investigating amygdala volumetry in patients with ASD compared with control subjects*

*Due to editorials guideline of limited number of references, only the most recent MRI studies on amygdala in ASD were considered, starting from year 2006.
‡Follow-up study; ASD, autism spectrum disorders; TD, typically developing control subjects; RD, subjects with reading disorders; HFA, high-functioning autism; LFA, low-functioning autism; AD, autistic disorder; AS, Asperger syndrome; ADM, autistic disorder with macrocephaly; TDM, typically developing control subjects with macrocephaly PAD, parents of children with autistic disorder; (m), males; (f), females; PDD-NOS, Pervasive developmental disorder not otherwise specified; PIQ, performance IQ; n.r., not reported; DD, developmental delay.
Interestingly, a correlation between the severity of core ASD symptoms and amygdala anatomy has been detected in several studies, with a different trajectory in accordance to age. Indeed, a direct correlation between amygdala volumes and degree of social and communicative impairment has been found in toddlers (Munson et al. Reference Munson, Dawson, Abbott, Faja, Webb, Friedman, Shaw, Artru and Dager2006; Schumann et al. Reference Schumann, Barnes, Lord and Courchesne2009), and younger children with ASD (Kim et al. Reference Kim, Lyoo, Estes, Renshaw, Shaw, Friedman, Kim, Yoon, Hwang and Dager2010). In contrast, smaller amygdalae associated with deficits of social reciprocity in older ASD children (Nacewitz et al. 2006) and with restricted-repetitive behaviour in adult subjects with Asperger syndrome (Dziobek et al. Reference Dziobek, Fleck, Rogers, Wolf and Convit2006).
In conclusion, there is evidence that amygdala volumes are enlarged in toddlers and younger children with ASD and correlate with social ability impairment. Nonetheless, some key issues remain to be clarified, specifically: (1) whether the onset of amygdala overgrowth in ASD is already present at birth or during the postnatal brain growth; (2) at which age the amygdala developmental trajectory decelerates in ASD, leading to attenuated differences with typically developing controls; (3) if gender and ASD phenotype (i.e., socio-communicative deficits) play a role on the above mentioned amygdala maturation. Only future prospective studies that follow over time, through multiple MRI scans, high-risk neonates well-characterized from the clinical point of view could provide insightful information into each of these research questions.
Acknowledgements
None.
Financial Support
S. C. was partly supported by the Italian Ministry of Health and by Tuscany Region with the grant ‘GR-2010-2317873′. F. M. and S. C. were partly supported by the European Union (The MICHELANGELO Project). The other authors received no specific grant from any funding agency, commercial or not-for-profit sectors for this publication.
Conflict of Interest
None.
Ethical Standards
The authors declare that no human or animal experimentation was conducted for this work.