Vigorous human-to-human transmission is one of the primary contributors to the establishment of pandemic influenza. Thus, understanding the dynamics of influenza viral load in the human upper respiratory tract can provide insight into the transmission and spread of the virus. The reported duration of viral shedding of A(H1N1)pdm09 is inconsistent between studies, ranging from 6 to 11 days [Reference Na1–Reference Malato3]. The virus has been shown to be shed 7 days after symptom onset in 9–20% of A(H1N1)pdm09 patients [Reference To4, Reference Meschi5]. Li et al. reported that 50% of A(H1N1)pdm09 influenza patients who were treated with oseltamivir over 2 days after symptom onset still had detectable virus 7 days after treatment initiation [Reference Li6].
The dynamics of viral load in patients with A(H1N1)pdm09 influenza has been investigated in a previous study, but the retrospective design of that study led to insufficient reliability due to missing samples caused by patient discharge and loss to follow-up [Reference Meschi5]. Our study was performed prospectively to investigate changes in viral load and the relationship between viral load and clinical findings in adult patients with A(H1N1)pdm09 influenza. Additionally, factors associated with prolonged viral shedding in the upper respiratory tract were examined.
A prospective study was performed on patients aged ⩾15 years with A(H1N1)pdm09 influenza. A nasopharyngeal or oropharyngeal swab was collected after obtaining written informed consent from patients who visited Korea University Guro Hospital, a 900-bed tertiary-care teaching hospital in Seoul, Korea, for influenza-like illness between August 2009 and January 2010. When a patient was confirmed to have A(H1N1)pdm09, two additional specimens were obtained serially at intervals of 2–4 days. A total of three samples per patient were collected within 10 days of confirmed influenza. Each time a specimen was collected, patients recorded their symptoms on a self-report questionnaire. Flocked swabs were kept in viral transport media at −70°C until use.
Viral loads were measured by quantitative real-time RT–PCR. Briefly, viral RNA was extracted using the ExiPrep™ Viral DNA/RNA Extraction kit (Bioneer, Korea). Real-time RT–PCR was performed using the AccuPower® New Influenza A(H1N1) Real-Time RT–PCR kit (Bioneer, Korea) and Exicycler™ 96 Real-Time Quantitative Thermal Block (Bioneer, Korea). A positive control with a known copy number was included to generate a standard curve for quantification. The detection limit was set at 100 copies/ml. Influenza viral loads were log transformed (log10 copies/ml).
The Mann–Whitney U test and Kruskal–Wallis test were used to analyse continuous variables and Fisher's exact test was adopted for categorical variables. Variables with P < 0·1 were incorporated into a multivariate logistic regression model. To analyse longitudinal data, a general linear model with correlated errors was adopted. Viral load was predicted using days after symptom onset, age group, hospitalization and the presence of clinical symptoms. Outliers were excluded from the fitting process. The analyses were performed using SAS v. 9.2 (SAS Institute Inc., USA) and a P value <0·05 was considered to indicate statistical significance. Ethical approval was obtained from the Institutional Review Board of the Hospital Authority of Korea University Guro Hospital (No.: GR0999-002).
Thirty-five patients with A(H1N1)pdm09 influenza who were treated with neuraminidase inhibitors were enrolled. A total of 105 serial flocked swabs were collected and analysed. Mean (±s.d.) age of the patients was 35·2 ± 16·9 years and 16 (45·7%) patients were male. Most patients (n = 29, 82·9%) were previously healthy without chronic medical diseases. Patients visited the hospital an average of 2·4 ± 2·0 days after symptom onset. Nine (25·7%) patients were hospitalized. The mean time to administration of an antiviral agent was 2·6 ± 1·8 days after symptom onset.
Mean A(H1N1)pdm09 viral load at the first hospital visit was 5·4 ± 1·5 log10 copies/ml. There were no significant differences between initial viral loads based on age group (15–29 years: 5·1 ± 1·7 log10 copies/ml; 30–49 years: 5·5 ± 1·4 log10 copies/ml; ⩾50 years: 5·9 ± 1·0 log10 copies/ml; P = 0·47). Initial influenza RNA concentrations in patients who visited the hospital within 48 h of symptom onset and those who visited 48 h after symptom onset were 5·6 ± 1·2 and 4·9 ± 2·0 log10 copies/ml, respectively (P = 0·41). There was also no difference in the initial viral load between hospitalized patients and outpatients (4·9 ± 2·1 vs. 5·6 ± 1·2 log10 copies/ml, P = 0·78).
Viral load peaked on the day of initial symptom onset and viral clearance was significantly correlated with passage of time from symptom onset (P < 0·01). Viral clearance differed according to age group (Fig. 1 a). The rate of viral clearance was significantly faster in patients aged 15–29 years than the other age groups (P < 0·01 for patients aged 30–49 years and ⩾50 years). There was no significant difference in the rate of viral clearance between patients aged 30–49 years and patients aged ⩾50 years (P = 0·11). The time to hospital visit from symptom onset did not differ significantly according to age group (15–29 years: 1·7 ± 1·0 days; 30–49 years: 2·9 ± 2·9 days; ⩾50 years: 3·1 ± 1·9 days; P = 0·12). Viral clearance was slower in hospitalized patients than in outpatients (P < 0·01) (Fig. 1 b). Hospitalized patients visited hospital significantly later after symptom onset than outpatients (3·7 ± 2·4 vs. 1·9 ± 1·6 days, P = 0·01). Patients who complained of cough (P = 0·02) and headache (P = 0·01) had higher viral loads in upper respiratory specimens than patients who did not complain of these symptoms (Fig. 1 c, d). Cough was found to be the most persistent symptom; 60·0% of patients complained of cough at their third hospital visit. More than 70% of patients complained of headache at their initial visit; however, the frequency declined to 8·6% at the third visit (Fig. 2).

Fig. 1. Viral clearance of A(H1N1)pdm09 influenza showed different patterns according to age. (a) The rate of viral clearance was faster in patients aged 15–29 years than other age groups (P < 0·01 for both patients aged 30–49 years and ⩾50 years). (b) Viral clearance was slower in hospitalized patients than in outpatients (P < 0·01). (c, d) Patients who complained of cough (P = 0·02) and headache (P = 0·01) had higher viral loads in their upper respiratory specimens than patients who did not complain of these symptoms.

Fig. 2. Self-reported symptoms were evaluated in patients with A(H1N1)pdm09 influenza. The frequencies with which patients complained of symptoms at their initial, second, and third visits, respectively, were as follows: (a) respiratory symptoms: cough (97·1%, 85·7%, 60·0%); sore throat (65·7%, 25·7%, 20·0%); rhinorrhoea (57·1%, 34·3%, 22·9%); nasal obstruction (37·1%, 25·7%, 14·3%); shortness of breath (40·0%, 8·6%, 5·7%); (b) general symptoms: headache (71·4%, 37·1%, 8·6%); myalgia (54·3%, 14·3%, 2·9%); fatigue (71·4%, 5·7%, 2·9%); chill (45·7%, 8·6%, 2·9%).
Prolonged viral shedding was found in 17 (48·6%) patients 5 days after symptom onset and in seven patients (20·0%) 7 days after symptom onset (Table 1). Prolonged viral shedding was significantly more frequent in patients who visited the hospital 48 h after symptom onset (52·9% vs. 47·1%, P = 0·01). Patients who received antiviral agents 48 h after symptom onset showed higher rates of viral shedding over the 5 days of illness than those patients who received antiviral agents within 48 h of symptom onset (58·8% vs. 41·2%, P = 0·09). The result of multivariate logistic regression indicated that the time to hospital visit from symptom onset (after 48 h) was a significant factor leading to prolonged shedding of A(H1N1)pdm09 influenza virus (odds ratio 9·0, 95% confidence interval 1·56–51·87, P = 0·01).
Table 1. Factors related to prolonged viral shedding after >5 days in patients with A(H1N1)pdm09 influenza
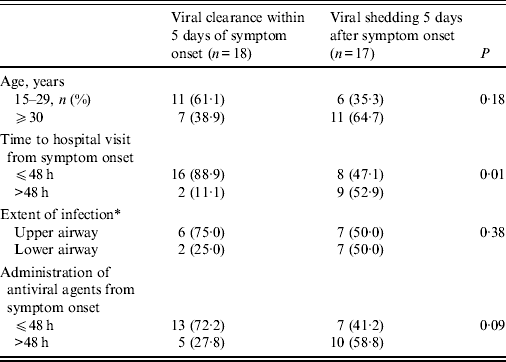
* Judged by radiological examination.
In this study, patients aged ⩾30 years had a slower rate of viral clearance in their upper respiratory specimens than patients aged 15–29 years. This result suggests that patients aged ⩾30 years may function as active viral shedders in the community. This information should be taken into consideration when designing social containment strategies for influenza outbreaks.
A clear relationship between age and influenza viral clearance has not previously been established. Advanced age (>65 years) was significantly associated with prolonged viral shedding in patients with influenza A(H3N2) [Reference Lee7]. However, two other studies reported that young age was associated with longer viral shedding of A(H1N1)pdm09 influenza [Reference To4, Reference Leung8]. However, these studies were performed in both children and adults, and it is difficult to interpret differences in viral clearance between younger and older adults. In an animal model, young mice showed higher pulmonary cytotoxic T lymphocyte (CTL) activity after H3N2 influenza challenge and higher CTL activity was considered to lead to rapid viral clearance [Reference Bender9]. Additionally, there was a delay in the maximal expansion of CD8+ lymphocytes after influenza A virus infection in aged mice that correlated with a delay in maximal cytotoxic activity and virus clearance [Reference Po10]. Thus, it is possible that a vigorous immune response in younger patients could lead to more rapid viral clearance. We did not include paediatric patients aged <15 years in this study. Further research into human immunity and clearance of the A(H1N1)pdm09 virus according to age is required.
In this study, viral clearance was correlated with resolution of cough and headache in patients with A(H1N1)pdm09 influenza. Lau et al. [Reference Lau11] found that tympanic temperature and a number of symptoms were positively correlated with viral shedding of seasonal influenza A. Li et al. [Reference Li6] reported that viral load was inversely correlated with lymphocyte count in patients with A(H1N1)pdm09 influenza, but no correlation was observed between viral load and number of symptoms. Most patients enrolled in our study were outpatients; therefore, it was not possible to analyse laboratory findings due to a lack of data. The time to administration of antiviral agents and lower respiratory tract involvement were not significantly correlated with viral clearance rate.
Previously, presence of pneumonia was suggested to result in longer detection of influenza virus RNA in nasopharyngeal swabs [Reference Meschi5]. Early oseltamivir treatment was related to a shorter duration of viral shedding in A(H1N1)pdm09 influenza [Reference Na1, Reference Leung8]. We found that the time to hospital visit after symptom onset was significantly correlated with prolonged viral shedding >5 days after symptom onset. There are two possible explanations for this finding. Antiviral agents were administered later after symptom onset in the prolonged viral shedding group than in those patients who did not shed the virus >5 days after symptom onset, even though the difference between these two groups was not statistically significant. Furthermore, there was no significant difference between the initial viral titres in patients who visited the hospital within 48 h of symptom onset compared to those who visited the hospital 48 h after symptom onset. Thus, there is a possibility that patients who had a longer duration of illness before they arrived at the hospital had higher viral titres at disease onset.
This study has some limitations. Sample size is small and paediatric patients were not included. Viral load was measured three times for each patient and the point of negative conversion of influenza virus in the respiratory tract was not determined accurately. For this reason, we could not determine the precise duration of viral shedding. Heterogeneous sample quality could have led to errors in estimation of the exact viral load. Furthermore, not all viruses detected by real-time RT–PCR are viable viruses. Thus, virus shedding by PCR may not reflect infectious virus, particularly during the late phase of illness. All patients were treated with neuraminidase inhibitors, which might affect the patterns of viral shedding and illness.
Nevertheless, this study is valuable for several reasons. Its prospective design provides a more accurate profile of the viral load dynamics of A(H1N1)pdm09 influenza. We also analysed the dynamics of clinical symptoms and correlated those with A(H1N1)pdm09 influenza viral load. While many other studies evaluated only hospitalized patients, we included outpatients in our analysis. An understanding of influenza viral load dynamics in outpatients is important, because these patients have a greater opportunity to shed influenza virus in the community than hospitalized patients. The information provided in this study will contribute to infection control aspects with respect to managing patients with influenza virus infections.
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.





