INTRODUCTION
Varicella-zoster virus (VZV) causes varicella which is one of the most contagious childhood diseases and the secondary infection rate from household contact can be as high as 90% [Reference Heininger and Seward1–4]. Although complications of varicella in childhood are rare, primary infection in pregnancy can cause serious feto-maternal complications. In temperate climates 96–98% of all women of childbearing age have antibodies against VZV [Reference Marin, Meissner and Seward5, Reference Liese6], with seroconversion occurring at the earliest age in Northern and Western Europe. Women from tropical countries and first-generation immigrants to Europe have a higher risk of being seronegative [Reference Svahn7–Reference Helmuth11].
The incidence of varicella in pregnancy is estimated to be about 1·6–4·6/1000 in an unvaccinated population in the UK and the United States [Reference Fairley and Miller12, Reference Seward, Galil and Wharton13]. The disease can be more severe in pregnant women than in non-pregnant adults and even with antiviral therapy the case-fatality rate has been reported to be 3–14% [Reference Stagno and Whitley14]. In particular, smokers and individuals with >100 vesicles [Reference Marin15, Reference Harger16] are at greater risk of varicella pneumonia (10–14% of cases) [Reference Gnann17]. The incidence of infection may be substantially higher for multiparous women than the overall incidence rates reported in adults due to higher risk of exposure from their own children [Reference Enders and Miller18].
Transmission of the virus from mother to fetus during the first and second trimester is of particular concern due to a small risk (1%) of congenital varicella syndrome which involves a range of anomalies and is associated with a high case-fatality rate (30%) [Reference Tan and Koren19–22]. The risk of transplacental infection increases with gestational age [Reference Lamont2, Reference Tan and Koren19] and neonatal varicella infection may arise if the mother is infected in late pregnancy. The risk is highest when infection occurs <5 days prior to and 2 days after delivery, before passive immunity from mother to baby can be conferred [Reference Miller, Cradock-Watson and Ridehalgh23]. Even with modern neonatal intensive care and medical treatment neonatal varicella is a serious illness with a mortality rate up to 30% [24].
Varicella-zoster immunoglobulin (VZIG) is a purified human immunoglobulin prepared from plasma with high varicella-zoster antibody titres [Reference Enders and Miller18]. In the 1960s immunoglobulin was shown to attenuate clinical infection in healthy children exposed to varicella. Later, in a double-blind controlled study, a product derived from plasma of donors with herpes zoster was shown to modify varicella in children with leukaemia [Reference Gershon, Takahashi, Seward, Plotkin, Orenstein and Offit25]. Observational studies have shown that VZIG in pregnant women reduces the risk of developing clinical disease and reduces the severity of the disease in those who develop symptoms in spite of VZIG treatment [Reference Enders and Miller18]. However, the effect of VZIG in pregnant women has never been tested in randomized controlled trials, and the effect on the fetus has not yet been established.
Ideally, a serological test should be conducted prior to administration of VZIG since most adults who recall no history of varicella are seropositive [Reference Alanen8]. Varicella vaccination is not recommended in pregnancy [26], but to avoid the risk of infection in future pregnancies postpartum immunization to susceptible women is recommended [Reference Bohlke27].
In Denmark, VZIG is not licensed for public sale, and is exclusively imported by the Statens Serum Institut (SSI). Since 2005, the provision of VZIG from SSI to Danish hospitals has been registered but the data has not been described previously, and information on the outcomes of the treatment has not been collected.
The aim of this study was to review VZIG provision formulas between December 2005 and March 2015 in order to:
-
• Describe indications for VZV prophylaxis by VZIG treatment in pregnancy by examining if the women who received VZIG differed from the general Danish population of women at childbearing age in relation to age, parity and country of origin.
-
• Identify the main sources of exposure as well as the time from exposure to treatment with VZIG.
-
• Follow-up on the women who received VZIG to examine if they developed primary varicella infection and if they received postpartum vaccination.
MATERIAL AND METHODS
This descriptive study consists of two parts: a register-based study and a follow-up study with information from a questionnaire sent to the participants. Data were obtained from three different sources: VZIG provision formulas from SSI, Danish registers, and a follow-up questionnaire.
VZIG provision formulas
According to Danish guidelines susceptible pregnant women with significant exposure to varicella are candidates for VZIG, which should be administrated preferably within 4 days and maximum 10 days after exposure [28]. As the supply of VZIG is scarce and treatment is expensive indications are considered thoroughly. According to current Danish guidelines [29] medical doctors should consult the 24/7 on-call service at the Department of Infectious Disease Epidemiology at SSI in order to request VZIG. At request of VZIG to a pregnant woman a doctor or nurse at SSI collects information on the date and nature of exposure and the results of the varicella antibody test. If there is indication for VZIG treatment a provision formula which includes information on the date of contact, the woman's civil registration number, gestational age, date and source of exposure, and name of the hospital and department is recorded. The VZIG dose is calculated and immediately dispatched to the hospital.
The VZIG provision formulas have been registered at SSI since December 2005 and we included all pregnant women who had been prescribed VZIG between December 2005 and March 2015.
Register data
The Civil Registration System (CRS). In Denmark all individuals are assigned a unique identification number, the civil registration number, which is recorded in the CRS. We collected information on date and country of birth, number of siblings, number of children and parity from CRS.
Statistics Denmark collects and publishes statistics on the Danish society. To compare the study population to the background population of women at reproductive age (15–45 years) data on country of origin as well as the number of women living in the five regions of Denmark was extracted from Statistics Denmark. The year of reference for the statistics was set to 2010: the middle of the study period [30].
The Danish National Register of Births. The SSI National Register of Births and Deliveries in Denmark [22] was used to compare the age of all women giving birth in Denmark in 2010. We also obtained information on the smoking habits for all women giving birth in Denmark in 2010.
Follow-up questionnaire
To obtain information on the course and outcome of the women's exposure to VZV a follow-up study was conducted. A structured and pilot tested questionnaire was designed and sent to all participants. The questionnaire included questions concerning events before, during and after exposure: history of having attended childcare institutions; profession, smoking habit and information on chronic diseases or medication at the time of exposure; development of clinical symptoms of primary VZV infection after VZIG treatment and vaccination after delivery (available from the corresponding author). The questionnaire was sent via mail and all participants were given 3 weeks to respond. If no response was received, telephone contact was attempted six times at different times of the day on different weekdays.
Statistical analysis
All data from VZIG provision formulas, data registers and follow-up questionnaire were entered into a common electronic database, ‘The VZIG Database’ using Microsoft Access 2013, and analysed using Microsoft Excel 2013 (Microsoft Corp., USA). Descriptive statistics were used to analyse the data. Categorical data was compared using a χ 2 test and statistical significance was defined as P < 0·05.
Ethical standards
The National Research Ethics Committee System was notified on the research project. It was decided that no ethical approval was necessary because biological samples were not used and the study included no biomedical intervention to humans. All participants who participated in the follow-up gave informed consent. The study was notified to The Danish Data Protection Agency (J. no. 2008-54-0472).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
A total of 106 VZIG provision formulas to pregnant women were registered at SSI from December 2005 to March 2015 and complete data (VZIG formula, CRS data and follow-up questionnaire) were available for 88 women. Inclusion of participants is summarized in Figure 1.

Fig. 1. Flowchart of the inclusion of 104 women treated with varicella-zoster immunoglobulin (VZIG) after exposure to varicella-zoster virus from December 2005 to March 2015. * Questionnaires not returned and telephone follow-up not possible due to missing contact information.
VZIG provision displayed seasonal variation with peaks in January and February and a second peak in May. The number dropped during the summer with fewest cases in August. The annual number of VZIG provisions gradually increased from 2006 to 2014 and peaked in 2014 with a total of 17 requests.
The geographical distribution of hospitals requesting VZIG is presented in Table 1. Based on the population size of women aged 15–45 years in each of the five regions in Denmark, the incidence of VZIG provision/100 000 was calculated. For the whole country, the incidence was 0·9/100 000 women per year (104 provisions in a population of 1 100 983 women aged 15–45 years). The incidence of VZIG provision was lower in the regions of Zealand and Southern Denmark than in the rest of the country (0·3 and 0·4/100 000 women of childbearing age per year, respectively).
Table 1. Incidence by region in VZIG provision from December 2005 to March 2015 in 104 pregnant women in Denmark
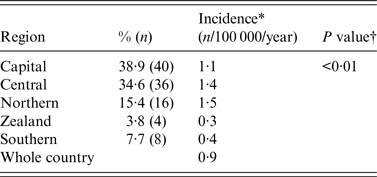
VZIG, Varicella-zoster immunoglobulin.
* Based on number of women aged 15–45 years in each region in Denmark. Data from Statistics Denmark, Statistikbanken, first quarter of 2010.
† χ 2 test for homogeneity.
The characteristics of women who received VZIG during pregnancy compared to the Danish background population of women at childbearing age are presented in Table 2. The mean age of the women in the study population was 30·6 years and there were most (43·3%) women in the 30–34 years age group. The parity differed significantly between the study population and the background population. A total of 57% of the women in the study population were second para compared to 37% in the background population. There were significantly more immigrants: 37·5% of the women came from other countries compared to 12% in the background population. The women had most often migrated to Denmark from Asia, South-Eastern Europe, and the Middle East.
Table 2. Characteristics of 104 women treated with VZIG during pregnancy from December 2005 to March 2015 compared to the Danish population of women aged 15–45 years
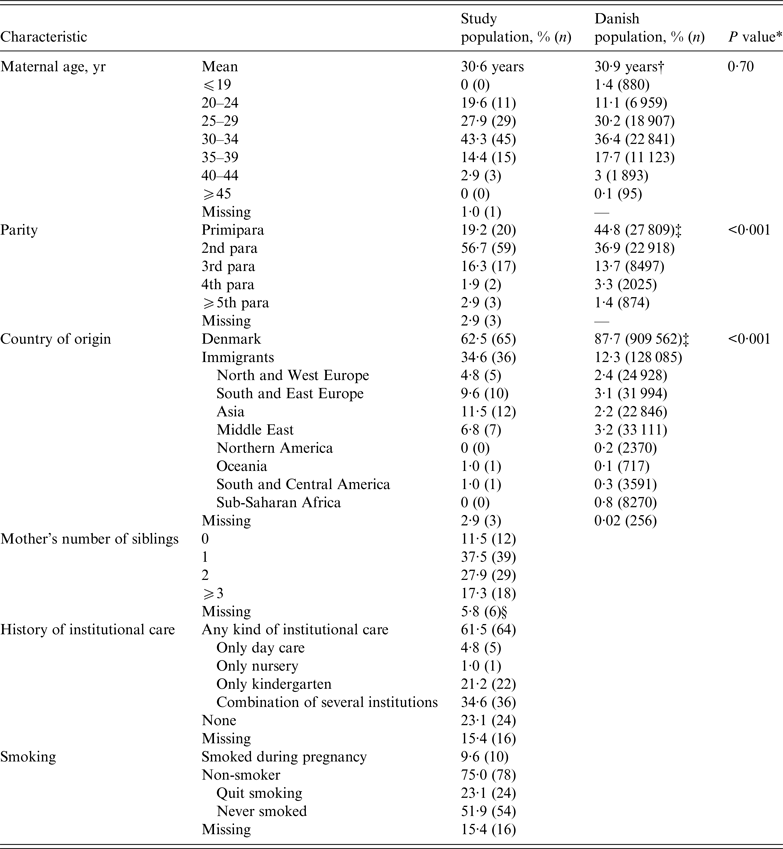
VZIG, Varicella-zoster immunoglobulin.
* χ 2 test for homogeneity.
† Mean age for women giving birth in Denmark 2010. Data from National Birth Statistics, 2012, Statens Serum Institut.
‡ Data from Statistics Denmark, Statistikbanken, 2010.
§ Missing values because the woman's parents never lived in Denmark and did not have a CRS number.
The women did not smoke more often during pregnancy than the background population (9·6% vs. 9·8%) [22]. Six (6·8%) women reported having a chronic disease during the pregnancy: ‘hypothyroidism’ (2), ‘gestational diabetes’ (1), ‘juvenile rheumatoid arthritis’ (1), ‘hepatitis B’ (1) and ‘a weak immune system’ (1) (not further specified by respondent).
The source of exposure to VZV was the woman's own child in 57·7% of the cases, and another child in 20% of the cases. All women who were exposed at their job worked in childcare institutions (n = 7). In total, 59 (57%) women received VZIG within 4 days of exposure to VZV and 40 (38·5%) women received VZIG between 4 and 10 days after exposure (Table 3).
Table 3. Characteristics of exposure to varicella-zoster virus in 104 pregnant women treated with VZIG from December 2005 to March 2015
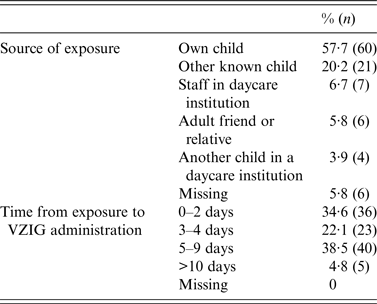
VZIG, Varicella-zoster immunoglobulin.
Missing values are due to missing information from the VZIG formula or from the questionnaire.
Of 88 women treated with VZIG that were followed up, five (6%) developed clinical symptoms of a primary VZV infection. Of these, three had received VZIG within 4 days of exposure and two developed a clinical infection.
The woman who reported the most severe symptoms of primary VZV infection was the one who received VZIG the longest time after exposure to the virus – 7 days. The woman reported developing more than 100 vesicles and had been hospitalized. She did not suffer from other medical illnesses at the time of infection, but developed eclampsia later in pregnancy. None of the women who developed clinical illness had complicated diseases with varicella pneumonia (Table 4), there were no deaths.
Table 4. Characteristics of five cases of primary varicella-zoster virus infection in pregnant women who received VZIG after exposure to varicella-zoster virus
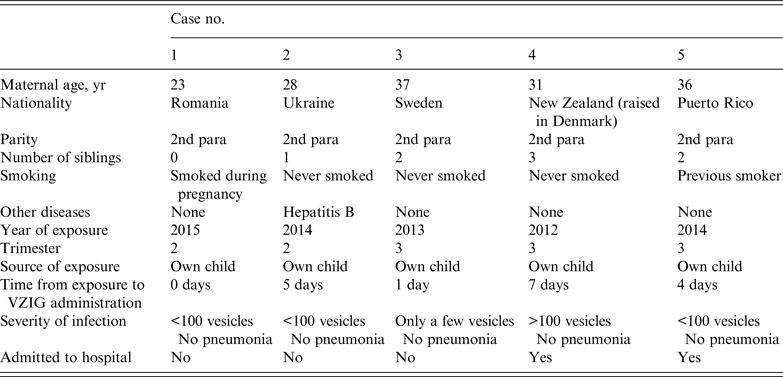
VZIG, Varicella-zoster immunoglobulin.
A total of 26·5% (22/83) of the women susceptible for infection reported receiving varicella vaccination after delivery.
DISCUSSION
In this national study of 104 pregnant women who received VZIG treatment over a 10-year period we describe characteristics and possible risk factors for VZIG treatment and follow-up to determine the outcome and number of breakthrough infections.
Our results confirm the results of previous epidemiological studies of VZV: VZV antibody-negative pregnant women who received VZIG treatment were more often immigrants, primarily from Asia and South-Eastern Europe, had the same mean age as the Danish background population of women at childbearing age, but were more often pregnant with their second child (57%) and had most commonly been exposed to VZV by their own child. All women that were exposed to VZV at their job worked in childcare institutions.
These findings underline that certain groups of women of childbearing age need increased attention: universal screening for varicella antibodies in women planning pregnancy has not proven to be cost-effective [Reference Manikkavasagan31], but general practitioners should be proactive in obtaining the history of varicella in women from non-temperate countries, especially if they are parous or if they work in childcare institutions. In case of a negative history of previous varicella infection the woman should be serotested, and if this is negative selective vaccination prior to pregnancy is recommended. If a seronegative woman becomes pregnant she needs to receive sufficient information about avoiding contact with individuals with varicella.
In this population, only 26·5% of the women reported receiving VZV vaccination after delivery. Offering VZV vaccination to seronegative women after delivery is crucial. The benefit of vaccination is threefold: preventing maternal disease and fetal infection in future pregnancies, thereby eliminating the woman's risk of going through the stressful treatment again, and minimizing the use of the expensive product, VZIG. Even though an economic model of postpartum vaccination of women who are seronegative for varicella indicates that it is cost-effective [Reference Pinot de Moira, Edmunds and Breuer32] varicella immunization is currently paid for privately by the patient.
Only five (6%) women developed varicella in spite of VZIG treatment, and all were exposed by their own child. This is lower than expected considering that VZV has a secondary infection rate from household contacts as high as 90% in individuals naive to the virus. The effectiveness of VZIG in pregnant women and in the fetus has not been established in randomized controlled studies for obvious ethical reasons. Previous research found that 50% of exposed pregnant women who received VZIG within 4 days of exposure developed clinical illness [Reference Enders and Miller18] compared to 7% (3/59) in our study. This difference could have several explanations. It could be concluded that VZIG treatment was more effective in preventing varicella infection in our study group, but different criteria for determining the antibody level could be another explanation. There are various commercial tests available for determining immunity to VZV by enzyme immunoassay (ELISA). These are known to vary in sensitivity and specificity. A low sensitivity of ELISA tests has been demonstrated in individuals known to be immune to VZV but who have tested negative for antibodies by commercial tests [Reference Gershon and Gershon33]. Thus, some of the women in our study with a negative serotest might in fact be immune to VZV. If this is the case, the reason that the woman does not develop varicella after exposure is that the woman is already immune to VZV and not because of the VZIG treatment. A number of different ELISA tests have been in use in Denmark over the 10-year period and we cannot exclude that a low sensitivity of the serological tests used may explain the low number of breakthrough infections in this study [Reference Nardone10].
However, as the women neither report any history of clinical varicella nor have positive serotests, it is not likely that this is the whole explanation for our findings.
Of the five women who developed varicella in spite of VZIG treatment only one reported a serious course of disease and no one developed pneumonia. In Scandinavia only Denmark routinely offers VZIG to pregnant women, whereas guidelines in Norway and Sweden do not include this expensive treatment to susceptible pregnant women exposed to VZV [34, Reference Johansen, Westergren and Lingaas35]. Studies that describe increased severity of varicella in pregnant women are older studies, and with the use of acyclovir and better intensive care the use of VZIG in pregnant women may be obsolete. However, the benefit of VZIG in the fetus is not known, and the possibility that VZIG can prevent congenital varicella syndrome might justify its further use.
Based on our results we cannot estimate the incidence of VZV infections in pregnant women but the average incidence of VZIG provision nationally was 0·9/100 000 per year. There was a geographical variation with lower requirements in the regions of Zealand and Southern Denmark. The difference may reflect a true difference in the distribution of seronegative women in the regions with more immigrants living in urban areas. Women in these regions might also pay less attention to exposure to varicella than women in the rest of the country and may not seek medical advice as often. The result may also reflect differences in the awareness of the indications for VZIG treatment among medical doctors in different regions.
We are not aware of previous studies that have described the outcome of VZIG treatments in pregnant women in Scandinavia. As VZIG is only available at SSI we assume that the VZIG provision registered reflects the actual number of VZIG treatments in Denmark. The completeness of this registration in combination with the information from CRS numbers formed a unique opportunity to describe women who received VZIG and the course of their pregnancy.
Since inclusion was based on VZIG formulas and CRS data, there were only a few missing cases with regard to these data. For the follow-up, contacting the women via telephone resulted in a high response rate.
However, even with a response rate on the follow-up questionnaire of 87%, a risk of selection bias in the population cannot be ignored. Most importantly, we know that all women (apart from the two that emigrated, for whom we have no data), were alive when the study was conducted, but we cannot exclude that some of the non-responders did develop varicella in their pregnancy which would result in an underestimation of the number of breakthrough infections. On the other hand women who developed varicella were likely be more motivated to participate in the study.
The population included women who were pregnant up to 10 years ago, and because we asked them to report the course of events there is a risk of recall bias. Some questions may be harder to answer than others, but we believe that having had a varicella infection with rash during pregnancy after having received VZIG at a hospital is such a significant event that most women are able to recall it, even many years later.
When considering parity and country of origin of the study group, we compared our study population to the background population of women of childbearing age in Denmark in the middle of the study period. It is a limitation that we did not include a proper control group of pregnant women who did not receive VZIG, matched by gestation and calendar period, which would have allowed a more robust evaluation of risk factors for VZIG treatment in pregnancy.
CONCLUSION
VZIG administration to pregnant Danish women is generally a rare event but when administered it seems effective in preventing varicella and also in preventing severe disease in those who in spite of VZIG administration develop primary varicella infection. General practitioners should be proactive in obtaining history on previous varicella infection in women of childbearing age from non-temperate countries in order to evaluate the need for selective pre-pregnancy varicella vaccination, and we recommend increased awareness on postpartum vaccination of seronegative women.
There is a need for more research to estimate the incidence and severity of varicella in pregnant women compared to non-pregnant adults and to uncover how many women in Denmark get varicella without previous VZIG administration. More studies are needed on the added value of using VZIG compared to antivirals for post-exposure prophylaxis.
DECLARATION OF INTEREST
I. G. Helmuth received research funding for a PhD study on varicella epidemiology in Denmark from vaccine manufacturer GlaxoSmithKline through an unrestricted grant to her employer at The Department of Paediatrics at Rigshospitalet.








