INTRODUCTION
Vancomycin-resistant enterococci (VRE) have been recognized as a leading cause of hospital-acquired infections (HAI) globally since its emergence in 1986 in the UK and France [Reference Uttley1]. VRE infections, with the organism's ability to acquire resistance and its intrinsic resistance to various antibiotics [Reference Murray2] coupled with the scarcity of reliable antimicrobial therapy for the effective treatment of serious bacteremia [Reference Landman and Quale3], present a clinical dilemma for infectious disease physicians. Enterococci are the third most common organism causing nosocomial bloodstream infections, with vancomycin resistance observed in 60% of Enterococcus faecium [Reference Wisplinghoff4].
Daptomycin is one of a limited number of antibiotics with effective bactericidal activity against VRE [Reference Canton5]. Soon after its approval for use by the US Food and Drug Administration in 2003, reports of infections with daptomycin non-susceptible enterococci (DNSE) emerged and cases have been described in patients both with and without prior daptomycin exposure [Reference Fraher6, Reference Kelesidis7]. The mechanism for the development of daptomycin non-susceptibility in enterococci has not been well elucidated [Reference Palmer8], although there is the suggestion that DNSE colonization or infection increased with increasing daptomycin use [Reference Storm9, Reference Kelesidis10].
In Singapore, daptomycin was approved for use in 2008 and utilization has increased over the years [Reference Hsu11]. Although daptomycin non-susceptible Staphylococcus aureus bacteraemia have been reported, little is known about the epidemiology of DNSE in Singapore and Asia. Since the first report of VRE in 1994, VRE rates have increased in Singapore, mirroring the pattern seen in Europe a decade earlier [Reference Molton12]. This study aims to determine the prevalence of DNSE in an Asian country with an increasing VRE prevalence and daptomycin use, and to understand the factors associated with reduced daptomycin susceptibility in VRE.
METHODS
We conducted a case-control study in a 1600-bed adult tertiary hospital in Singapore. The prevalence of clinical VRE infections in the hospital increased from 9·97/100 000 deaths and discharges in 2010 to 77·11/100 000 in 2012. Correspondingly, daptomycin utilization increased by 6·4-fold from an average monthly defined daily dose/1000 patient-days of 0·2 in 2010 to 1·28 in 2012. The hospital had an active VRE screening programme where surveillance cultures were taken from ‘high-risk’ patients who were on renal dialysis or had a history of prior hospitalization in a foreign or private hospital in the preceding 12 months, on admission to the hospital. In addition, whenever a VRE infection was detected in an inpatient, contact screening of all patients who were in the same ward as the index patient for the duration of the patient's hospital stay prior to isolation was carried out.
Clinical and surveillance samples were plated on selective medium chromID VRE plates (bioMérieux, USA) and incubated aerobically at 35–37 °C for 48 h.
Suspect colonies were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonik GmHB, Germany); antimicrobial susceptibility testing was performed for ampicillin, ciprofloxacin, clindamycin, erythromycin, gentamicin (high level), linezolid, minocycline, penicillin, teicoplanin, and vancomycin, using the Vitek 2 System (bioMérieux) [Reference Abele-Horn13]. Select strains that were identified with low discrimination were confirmed using an in-house multiplex polymerase chain reaction based on primer sets published by Dutka-Malen et al. [Reference Dutka-Malen, Evers and Courvalin14].
All VRE isolates from patients hospitalized from 1 January to 31 December 2012 were tested for daptomycin susceptibility using the Etest (bioMérieux). We defined daptomycin-reduced susceptible VRE (DRS-VRE), by using the criteria of the Clinical and Laboratory Standards Institute [15], as patients with a VRE isolate with daptomycin minimum inhibitory concentration (MIC) ⩾3 µg/ml from screening or clinical cultures. Daptomycin-susceptible VRE (DS-VRE) was defined as patients with VRE isolates with daptomycin MIC <3 µg/ml. For patients with VRE isolated from multiple samples, we only included the first VRE isolate and examined exposures prior to the first isolation of VRE.
We reviewed medical records for clinical and epidemiological data, and recorded patients' demographics, comorbidities, prior healthcare exposures, current hospitalization experiences, antibiotic exposures and durations within the past 3 months, antimicrobial susceptibilities and genotypes of VRE. Additionally, Charlson's comorbidity index [Reference Charlson16] was derived using coding algorithms. Clinical infection was determined as part of the hospital infection control unit's routine workflow, after review of patients for signs and symptoms of infection based on guidelines by the Centers for Disease Control and Prevention's National Healthcare Safety Network.
We computed odds ratios (ORs) and 95% confidence intervals (CIs), to compare differences in exposures and covariates between DRS-VRE and DS-VRE. Next, we constructed a multiple logistic regression model to control for confounding and assess for independent factors associated with DRS-VRE colonization or infection. We included variables decided a priori as factors associated with reduced daptomycin susceptibility based on prior knowledge from literature review to be associated with DNSE colonization and infection. We used the Hosmer–Lemeshow test to check for goodness of fit for the model. All statistical analyses were performed using Stata v. 12 (Stata Corp., USA).
Ethical approval
This study was approved by the Domain Specific Review Board of the National Healthcare Group, Singapore (DSRB- 2013/00228).
RESULTS
In total, 243 VRE isolates were analysed, including both VanA (133, 55%) and VanB (110, 45%) genotypes. The majority of VREs (213, 88%) were isolated from screening cultures. Of the VRE clinical infections, urine was found to be the main source (67%). The daptomycin MICs were distributed similarly between genotypes (Fig. 1).
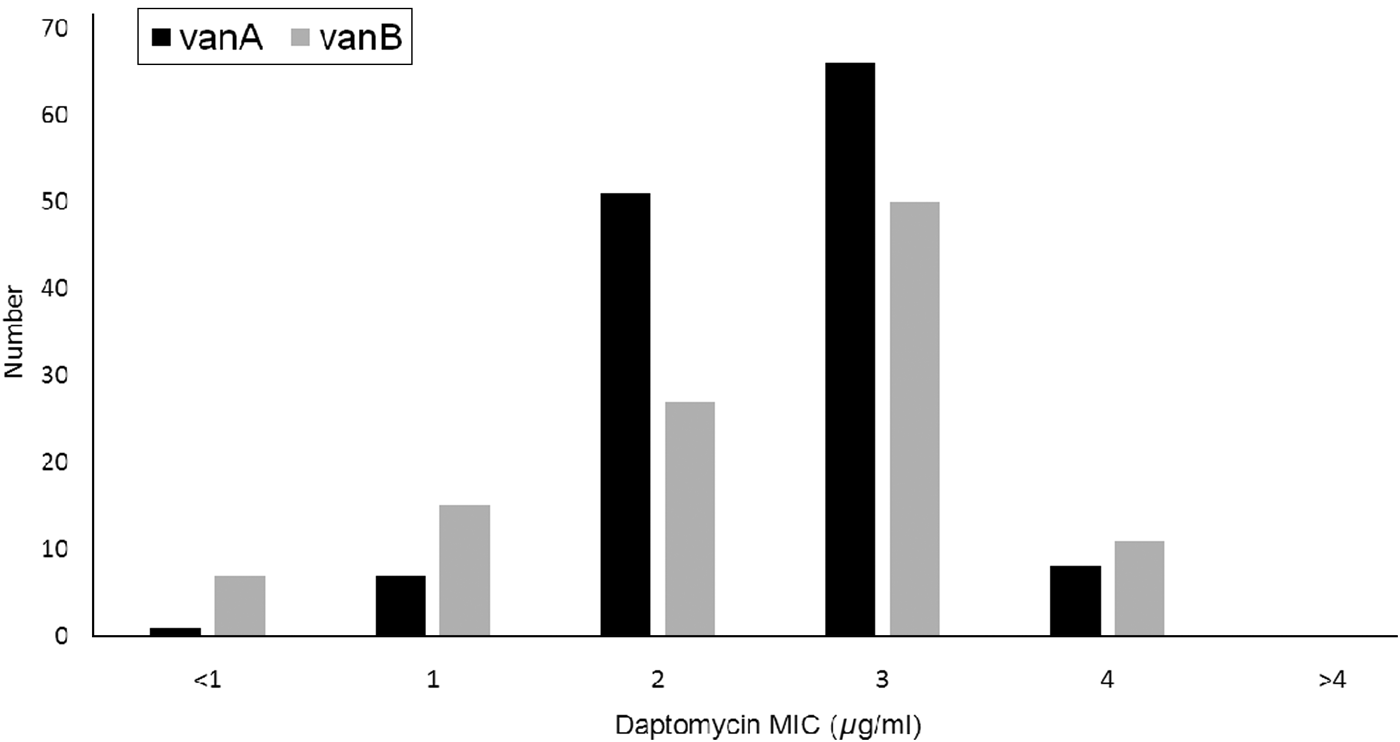
Fig. 1. Daptomycin minimum inhibitory concentration (MIC) levels by vancomycin-resistant enterococcus genotype.
None of the isolates was determined to be daptomycin non-susceptible based on the Clinical Laboratory Standards Institute's antimicrobial susceptibility testing standards of MIC >4 µg/ml [15]. However, about half of the patients (135, 55%) had reduced susceptibility to daptomycin with MIC 3–4 µg/ml. Two-thirds (20/30) of clinical isolates had reduced daptomycin susceptibility compared to 54% (115/213) of screening isolates (OR 1·70, 95% CI 0·72–4·27, P = 0·191).
There was no difference in age and gender between the DRS-VRE and DS-VRE groups (Table 1). Both groups had similar prior healthcare exposures, with more than 70% having been hospitalized in the preceding year. Of the 135 DRS-VRE patients, more than two-thirds (67·4%) had a Charlson comorbidity score of ⩾3 and 76·3% were immunosuppressed. The mean duration of hospital stay for all VRE patients was 26 days. Of note, DRS-VRE isolates (14·8%) were more likely to be from clinical infections than DS-VRE isolates (9·3%). Interestingly, patients who had >1 movement between wards during the hospitalization episode of VRE isolation (OR 0·57, 95% CI 0·33–0·98, P = 0·040) were less likely to have DRS-VRE. Compared to DRS-VRE, DS-VRE patients were more likely to have experienced >1 movement between beds and to have been exposed to indwelling devices during hospitalization.
Table 1. Univariate and multivariate analyses of risk factors associated with daptomycin-reduced susceptible vancomycin-resistant enterococci (DRS-VRE)
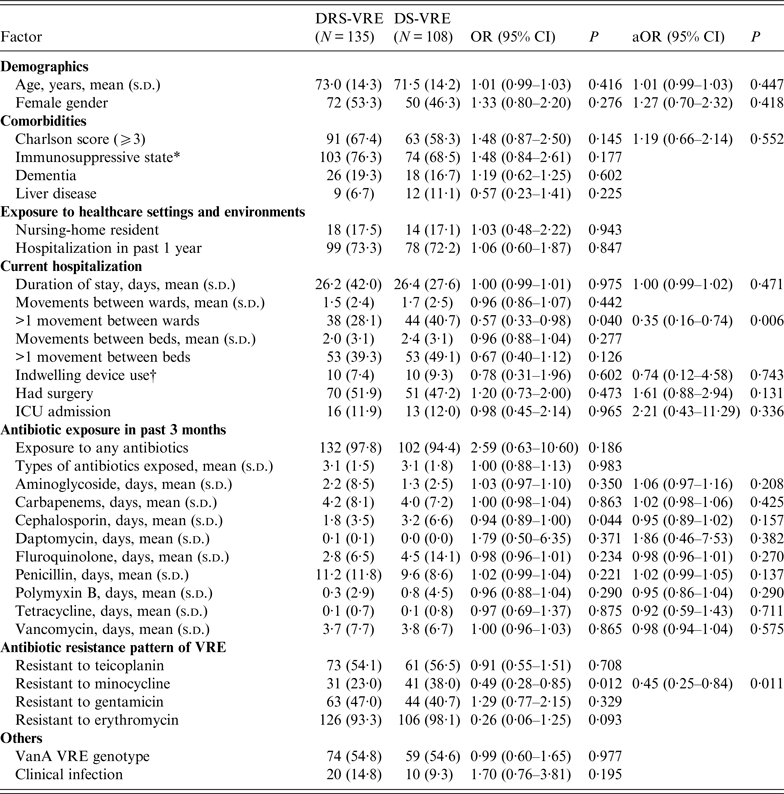
OR, Odds ratio; aOR, adjusted odds ratio; CI, confidence interval.
Values are n (%) unless otherwise indicated.
The percentage is of patients for whom data were available, i.e. excluding the missing values.
* Includes one or more of the following: diabetes with and without chronic complication, leukaemia, lymphoma, any malignancy without metastases, metastatic solid tumour, connective tissue disease, HIV, renal disease.
† Central venous pressure line, endotracheal tube, peripherally inserted central catheter, urinary catheterization.
None of the DS-VRE patients in the study cohort had been exposed to daptomycin in the preceding 3 months, compared to three of the DRS-VRE patients. An antimicrobial exposure in the prior 3 months was not significantly different between the two groups, with exposure to penicillin being the longest. Duration of exposure to cephalosporin (OR 0·94, 95% CI 0·89–1·00, P = 0·044) was observed to be protective against DRS-VRE. Of note, VRE isolates that were resistant to minocycline were half as likely to have reduced daptomycin susceptibility as those who were not (OR 0·49, 95% CI 0·28–0·85, P = 0·012). In both DRS-VRE and DS-VRE, >90% of VRE isolates were resistant to erythromycin.
There was no difference in VRE genotypes, hospitalization exposure in the prior 1 year, number of movements between beds during hospitalization, number of types of antibiotics exposed to, exposure to surgical interventions, and admission to the intensive care unit, between DRS-VRE and DS-VRE patients. Four (20%) out of the 20 DRS-VRE infected patients died during the hospitalization, compared to one (10%) out of 10 DS-VRE patients [OR 2·00, 95% CI 0·26–15·62, P = 0·640 (Fisher's exact test)].
In multivariate analyses, the two independent factors associated with DRS-VRE isolation were >1 movement between wards (OR 0·35, 95% CI 0·16–0·74, P = 0·006) and resistance to minocycline (OR 0·45, 95% CI 0·25–0·84, P = 0·011), after adjusting for age, gender, Charlson's comorbidity score ⩾3, duration of hospital stay, history of surgery, presence of indwelling devices, and duration of antibiotic exposure (to aminoglycoside, carbapenem, cephalosporin, daptomycin, fluoroquinolone, penicillin, polymyxin B, tetracycline, vancomycin) in the prior 3 months.
DISCUSSION
Limited data is available on the risk factors associated with DNSE. To the best of our knowledge, this is the first study in Asia to assess the epidemiology of DNSE and factors associated with reduced daptomycin susceptibility in VRE. Daptomycin non-susceptibility (MIC >4) was not observed in our patient cohort, although 55% had reduced susceptibility to daptomycin (MIC 3–4 µg/ml). We observed similar daptomycin resistance patterns in genotypes VanA and VanB. A surveillance study on VRE in Taiwan during the same period also did not report any daptomycin non-susceptibility [Reference Tse17]. The importance of reduced susceptibility to daptomycin has been under-recognized. The presence of mutations associated with daptomycin resistance have been recently observed in VRE isolates with daptomycin MIC 3–4 µg/ml, raising concerns about the effectiveness of daptomycin therapy in patients with such VRE infections [Reference McKinnell and Arias18]. Enterococcal isolates with daptomycin MIC 3–4 µg/ml have been observed to harbour mutations in liaFSR, a three-component regulatory system controlling cell-envelope stress response, that have been linked with the emergence of daptomycin resistance in enterococci [Reference Munita19]. Another competing pathway associated with yycFGHIJ mutations have been reported to play an important role in the successful treatment of DRS-VRE infections and prevention of development into daptomycin non-susceptibility (MIC >4 µg/ml) [Reference Humphries20].
In our study, we observed a relatively strong association between the duration of daptomycin exposure and daptomycin-reduced susceptibility (OR 1·86, 95% CI 0·46–7·53), although statistical significance could not be reached due to the limited number of exposed patients. Another study conducted at the Detroit Medical Center similarly could not conclude that prior daptomycin exposure was a predictor for DNSE, due to the rare occurrence of prior daptomycin exposure [Reference Judge21]. Our findings support the observations of an earlier study at the University of Iowa Hospitals and Clinics which suggested the emergence of daptomycin non-susceptibility under the antimicrobial pressure of prior daptomycin exposure [Reference Storm9]. In that study, the number of patients with DNSE colonization or infection increased rapidly with the surge in daptomycin use.
Although immunosuppression and multiple comorbid conditions were reported to be associated with DNSE [Reference Storm9, Reference Judge21], they were not observed to be associated with DRS-VRE in our study cohort. Our findings suggest that an immunosuppressive state might not be necessary for the development of daptomycin-reduced susceptibility. We also did not observe an increased risk of DRS-VRE in patients with indwelling devices, although exposure to such devices was previously reported to be an independent risk factor for DNSE [Reference Wisplinghoff4].
Interestingly, we observed that VRE isolates that were resistant to minocycline were negatively associated with reduced daptomycin susceptibility. Our observation corroborates the finding of a recent study which reported minocycline resistance in VRE isolates that were susceptible to daptomycin from infected urinary stents in renal transplant patients [Reference Descourouez22]. Further studies are needed to better understand the mechanisms for antimicrobial resistance.
In our study, patients who had moved between wards were less likely to have DRS-VRE than those who had not (OR 0·35, 95% CI 0·16–0·74). This was not previously reported by studies on DNSE and more studies are warranted before any conclusions can be made.
Our study may have been limited by the small number of patients with prior daptomycin exposure. Nonetheless, we identified some interesting factors associated with reduced daptomycin susceptibility in VRE in an Asian country with increasing VRE prevalence and daptomycin use. Our findings suggest an association between daptomycin exposure and reduced daptomycin susceptibility.
CONCLUSIONS
DNSE was not observed in our study population; however, 55% had reduced daptomycin susceptibility with MIC 3–4 µg/ml. Such reductions in susceptibility to daptomycin have important clinical implications as mutations associated with daptomycin resistance have been observed. Our study suggests that daptomycin exposure, movement between wards, and resistance to minocycline were associated with reduced daptomycin susceptibility in VRE. Active surveillance for reduced susceptibility and non-susceptibility to daptomycin in VRE colonized or infected patients with prior daptomycin therapy is crucial, as the use of daptomycin increases against the backdrop of the VRE surge in Asia.
ACKNOWLEDGEMENTS
This study is supported by the Society of Infectious Diseases Singapore (SIDS)'s inaugural research fund.
DECLARATION OF INTEREST
None.




