INTRODUCTION
Norovirus is the leading cause of acute gastroenteritis in the USA [Reference Hall1]. This extensive disease burden occurs due to a number of mechanisms resulting in a highly infectious – though relatively mild and self-limiting – pathogen [Reference Hall2]. Norovirus outbreaks are common with environmental, foodborne, person-to-person and waterborne modes of transmission being well documented [Reference Bitler3]. Norovirus human challenge studies have indicated that as few as 18–2800 viral particles are necessary to initiate infection [Reference Teunis4, Reference Atmar5]. Norovirus is also quite environmentally stable and resistant to many common disinfectants [Reference Lopman6]. In addition, norovirus has many genotypes and limited cross-protection from subsequent infection with different genotypes appears to exist [Reference Wyatt7].
To add to the challenge of controlling and preventing the spread of norovirus, there are currently no available vaccines or antivirals on the market, yet multiple vaccine candidates are currently under development [Reference Baehner, Bogaerts and Goodwin8]. A target population of interest includes individuals living in close proximity to others, such as those in military barracks, university dormitories and nursing homes [Reference Heusinkveld9–Reference Rha11]. Since children are also a potential target population for future vaccine trials, understanding household transmission is particularly important to predict the potential impacts of vaccination [Reference Aliabadi12].
Although norovirus transmission is commonly reported in households, little is known about the transmission dynamics and risk factors associated with household transmission of norovirus [Reference Aliabadi12–Reference Gastanaduy15]. To develop better control and prevention strategies in this setting, a greater understanding of the risk factors associated with household transmission of norovirus is needed. The objective of this study was to identify risk factors for susceptibility and infectiousness that facilitate secondary household transmission of norovirus in outbreak settings. We examined three norovirus outbreaks with reported secondary household transmission and developed statistical models to identify susceptibility and infectiousness risk factors associated with secondary household transmission of norovirus.
METHODS
Three norovirus outbreaks were investigated and data were collected on individuals exposed in the primary outbreak setting and their household members. The three outbreaks included two restaurant outbreaks and one high school outbreak.
Outbreak descriptions
Outbreak 1 occurred in a North Carolina restaurant in December 2009 and is described in more detail by Alfano-Sobsey et al. [Reference Alfano-Sobsey13]. Three stool specimens from cases contained laboratory evidence of norovirus infection, two of which could be sequenced yielding identical genotype GII.12 sequences, and an implicated lot of oysters was identified and subsequently recalled. Outbreak 2 occurred during July–August 2012 at a Michigan restaurant that additionally provided food to two public events. Five cases had laboratory-confirmed norovirus infections, including one restaurant worker, and these were subsequently genotyped as GII.6. Outbreak 3 occurred in November 2012 in Michigan among students at a high school. A preliminary investigation was conducted and three ill individuals were laboratory confirmed to be infected with norovirus, genotype GI.6. One high school kitchen worker was identified as being ill one week prior to the outbreak; no stool specimen was collected from this worker.
Secondary household transmission assessment
Potentially exposed individuals from each of the three outbreaks were interviewed by phone using a brief, scripted questionnaire. For Outbreak 1, an individual was interviewed if they attended the restaurant during the outbreak at-risk period of interest, 10–31 December 2009, and if they had not already been interviewed for the case–control study. For outbreak 2, an individual was interviewed if they reported dining at one of three locations during the outbreak at-risk period of interest, 23–28 July 2012, and individuals were contacted for interviews during 10–16 August 2012. For Outbreak 3, staff and parents of students were interviewed if they attended the high school during the outbreak at-risk period of interest, 12–16 November 2012, and individuals were contacted for interviews during 7–21 December 2012. Individuals reporting multiple dining events (for outbreaks 1 and 2) within the outbreak-specific at-risk period of interest were excluded due to uncertainty about the timing of their primary exposure. During interviews, information on all members of the household was collected, including age; sex; primary exposure history (i.e., dining at restaurants associated with outbreak 1 or 2 or presence at the high school associated with outbreak 3 during the respective at-risk periods of interest); date and time of day of illness onset and resolution; and specific symptoms. When a household had more than one primary case, the average of the age of the primary cases was taken. Symptoms for which information was collected included abdominal pain, diarrhoea, fever, headache, nausea and vomiting. Vomiting and diarrhoea were collected as zero, one, or multiple episodes, while the presence or absence of abdominal pain, fever, headache, and nausea were recorded. An individual was classified as having vomiting if they reported one or multiple vomiting events and classified as having diarrhoea if they reported one or multiple diarrhoeal events. In this study, an ill individual was defined as a person who experienced at least one episode of vomiting or diarrhoea. Households were recorded as non-responders after three unsuccessful attempts by phone contact.
Analysis
Information on date of exposure and date of illness onset was used to define individual case status. Date of illness onset and recovery were collected as the event date and dichotomous time of day (i.e., morning [AM] or evening [PM]). A primary case was defined as an individual present at a primary exposure location during the at-risk period of interest (10–31 December 2009 for outbreak 1, 23–28 July 2012 for outbreak 2 and 10–16 August 2012 for outbreak 3) and who reported illness onset within three days of exposure. A secondary case was defined as: (1) an individual who was not present at the primary exposure location during the at-risk period of interest, had contact with a primary case, and reported illness onset up to 14 days after illness onset in a household primary case or (2) an individual who was present at a primary exposure location within the at-risk period of interest, had contact with a primary case, and reported illness onset four or more days following that exposure. A household met the study inclusion criteria if there were at least two members in the household, at least one household member was a primary case, and at least one household contact was exposed to a primary case but was not present at a primary exposure location during the at-risk period of interest.
The secondary attack rate (SAR) was calculated as the proportion of secondary cases among the household contacts. We examined risk factors for infectiousness and susceptibility by comparing characteristics (household, clinical, and demographic) of primary cases and other household members, respectively. Chi-square tests were conducted for all categorical risk factors. Normality of continuous risk factors was assessed using the Kolmogrov–Smirnov test. All non-normally distributed risk factors were assessed using the Mann–Whitney U-test. To account for correlation of characteristics among individuals from the same household, potential susceptibility and infectiousness risk factors were assessed using univariate and multivariate generalised linear mixed models. To account for potential confounding effects from combining data from three different outbreaks, a hierarchical control term for the outbreak was included in all univariate and multivariate models. Additional potential confounding effects were addressed in multivariate models by including all risk factors with P-values <0·1 in the univariate models. All analyses were conducted in the statistical software package, SAS 9·4 (SAS Institute, Inc., Cary, NC).
RESULTS
Contact was attempted with a member associated with 105 households from outbreak 1, of whom 78 (74%) were interviewed; nine (12%) of these households were subsequently excluded due to multiple visits, dining outside the at-risk period of interest, not having a primary case, or for being a single member household. From outbreak 2, contact was attempted with a household member associated with 198 households, of whom 118 (60%) were interviewed; 73 (37%) households could not be reached and seven (4%) households refused to be interviewed. Interviews were attempted for 179 households in outbreak 3, of which 52 (29%) were interviewed; 121 (68%) households could not be reached and six (3%) households refused to be interviewed. Ill individuals who could not be contacted were not significantly different from ill individuals who were contacted, when compared by age, sex, presentation of vomiting or diarrhoea, and seeking of medical care (data not shown). Moreover, the SARs were compared for the three outbreaks and were not significantly different (outbreak 1: 16%, outbreak 2: 20%, outbreak 3: 22%; P = 0·60). Regardless of including or excluding the outbreak effects term in the models, there was no significant change in the model estimates (data not shown).
One hundred and forty-six households, containing 538 individuals, met the inclusion criteria and were included in the analyses. Of these, 220 (41%) individuals reported exposure at one of three primary exposure locations, of which 185 were categorised as primary cases (Table 1). Secondary transmission was reported in 36 (25%) of households, which included 52 primary cases (16 in outbreak 1, 23 in outbreak 2, and 13 in outbreak 3). The median household size was not significantly different in households with transmission compared to those without (4 vs. 3, P = 0·08). Moreover, the distribution of primary case symptoms was not significantly different among those living in households with secondary transmission compared with those without (abdominal pain (74% vs. 79%), fever (58% vs. 49%), headache (65% vs. 70%), nausea (85% vs. 90%) and vomiting (83% vs. 79%), respectively), yet more primary cases reported diarrhoea in transmission households compared with those without (98% vs. 81%, P = 0·01). Finally, median age (32 years (range: 14–65) vs. 34 years (range: 14–87), P = 0·59) and illness duration (1·5 days (range: 0·25–13) vs. 1·5 days (range: 0·25–14), P = 0·46) were not significantly different among primary cases living in households with transmission compared with those without.
Table 1. Individual and household-level characteristics of three norovirus outbreaks included in the study

* An individual who attended a primary exposure location.
† An individual who was exposed to a primary case but did not attend a primary exposure location.
‡ Secondary attack rate calculated by dividing the number of secondary cases by the number of household contacts.
|| Information available on illness duration for 100, 74 and 68 participants from Outbreaks 1, 2 and 3, respectively.
¶ A household with at least one primary case and one secondary case illness onset within 14 days of primary case illness onset.
Among the 331 household contacts (i.e., individuals not present at a primary exposure location), a total of 66 secondary cases were reported leading to an overall SAR of 20% (66/331). Symptoms of secondary cases included nausea (88%), diarrhoea (85%), vomiting (83%), abdominal pain (82%), headache (73%), and fever (56%). The median time from earliest household primary case exposure to secondary case illness onset was 5 days (range: 2–14 days, Fig. 1). Additionally, the median time between illness onset of the first primary case in a given household and the first secondary case was 2·5 days (range: 0·5–11 days).

Fig. 1. Distribution of primary and secondary case illness onset. Illness onset calculated as the number of days from the exposure date of the first household primary case at a primary exposure location to the date of self-reported illness onset (Primary cases: n = 132, Secondary cases: n = 35; missing cases were due to no reported exposure date by the household primary case).
The effect of household-level infectiousness and individual-level susceptibility risk factors on the SAR is shown in Table 2. The SAR was significantly higher among those living in households with two or more primary cases compared with living in a household with one primary case (incidence rate ratio (IRR) = 2·1; 95% confidence interval (CI) 1·37–3·29). In addition, households with two or more primary cases with vomiting had a SAR nearly twice as high as households with one or no primary cases with vomiting (IRR = 1·9; 95% CI 1·11–3·37). Finally, living in a household with at least one primary case with diarrhoea resulted in a significantly higher SAR compared with living in a household with no primary cases with diarrhoea (IRR = 3·0; 95% CI 1·46–6·01).
Table 2. Results of univariate and multivariate generalised linear mixed modelling of norovirus secondary household transmission
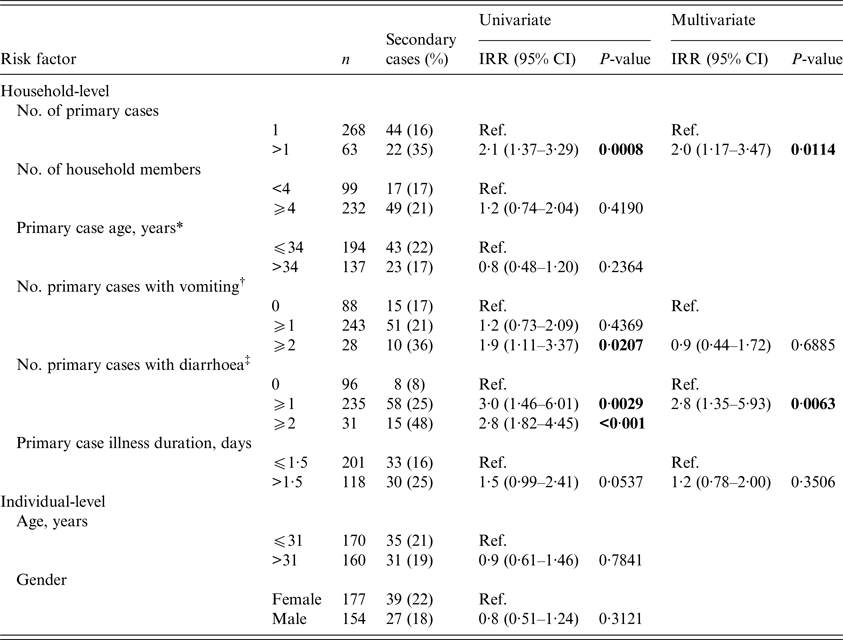
* If more than one primary case resides in the same household the average of the ages was taken.
† Vomiting was defined as an individual who reported one or multiple vomiting events.
‡ Diarrhoea was defined as an individual who reported one or multiple diarrhoea events.
Four risk factors were included in the multivariate analysis: two or more primary cases, two or more primary cases with vomiting, at least one primary case with diarrhoea, and primary case illness duration (Table 2). In the multivariate model, the SAR was significantly higher among those living in households with two or more household primary cases compared with those with one (adjusted IRR = 2·0; 95% CI 1·17–3·47) and among those living in households with at least one primary case with diarrhoea compared with none (adjusted IRR = 2·8; 95% CI 1·35–5·93).
DISCUSSION
The results presented in this study highlight the infectious nature of norovirus in the household and identify risk factors that facilitate secondary transmission. Across the three outbreaks, a quarter of surveyed households reported secondary household transmission. The household transmission rate in this study is relatively consistent with previous accounts of norovirus secondary transmission in this setting (44% in Heun et al.; 33% in Gastañaduy et al.; 19% in Baron et al.); the somewhat higher household transmission rates in some of the other studies may be explained by differences in primary case demographics [Reference Heun14–Reference Baron16]. Young children have been described previously as a high-risk group in terms of transmissibility [Reference Aliabadi12, Reference de Wit, Koopmans and van Duynhoven17]. Therefore, the higher proportion of young primary cases in other studies could explain the higher household transmission rates compared to this study where the median primary case age was 34 [Reference Heun14, Reference Gastanaduy15]. Additionally, one of the studies was conducted in Ecuador and may reflect different sociodemographic, sanitation, or hygiene conditions that may have also contributed to a higher household transmission rate [Reference Gastanaduy15].
After evaluating many risk factors, two risk factors were significantly associated with secondary household transmission: living in a household with: (1) two or more primary cases and (2) at least one primary case with diarrhoea. Although this study included data from three outbreaks each with a different genotype, the effect of the risk factors on the SAR was the same. This is important because it suggests the recommended control measures in this study may be generalisable despite the fact that different norovirus genotypes are more commonly associated certain settings and modes of transmission [Reference Leshem18, Reference Vega and Vinje19]. The effect of the number of household primary cases on norovirus household transmission has been observed previously [Reference de Wit, Koopmans and van Duynhoven17]. This association seems logical since a greater number of norovirus primary cases increases the number of sources from which highly infectious virus could spread. In addition to the number of primary cases, living with primary cases reporting diarrhoea also increased the SAR, which is consistent with previous reports implicating diarrhoea in potentiating household transmission [Reference de Wit, Koopmans and van Duynhoven17]. Importantly, this study was able to demonstrate that the number of primary cases with diarrhoea had an effect on secondary household transmission of norovirus.
In this study, living with at least one primary case with diarrhoea may have significantly increased household transmission in part due to the dynamics of viral shedding. The duration of norovirus shedding is long and generally continues beyond the symptomatic period [Reference Teunis20, Reference Kirby21]. Furthermore, the viral titres shed in the stool of symptomatic and asymptomatic individuals are extremely high, though the titres in those without symptoms tend to be lower [Reference Kirby21]. Even so, the viral titres of asymptomatic individuals are generally thought to be high enough to produce additional cases due to the low infectious dose of norovirus [Reference Teunis4, Reference Atmar5]. As Figure 1 shows, certain secondary cases reported illness onset nearly two weeks after first household primary case exposure, which is longer than the typical incubation period for norovirus. Thus, these secondary cases may have resulted from transmission from asymptomatic household members infected at the primary exposure setting or by post-symptomatic primary cases. This has been clearly demonstrated in foodborne outbreaks of norovirus, in which post-symptomatic food handlers were implicated as the source of infection [Reference Franck22, Reference Thornley23]. Presumably, the persistence of viral shedding after symptom resolution increases the challenge of preventing further spread since most individuals associate contagiousness with the presence of symptoms. As a result, individuals – even those who practiced good hygiene during their symptomatic period – may still contribute to secondary household transmission due to prolonged asymptomatic shedding.
Primary cases with diarrhoea may have also inadvertently caused environmental dispersion and subsequent persistence of norovirus in the household [Reference Bonifait24]. Environmental contamination of the household may be an alternative explanation for the prolonged illness onset observed among a group of secondary cases in Figure 1. This environmental contamination may, in part, have contributed to the observed increased rate of secondary household transmission, particularly when there was more than one primary case with diarrhoea in a household. In addition to being easily transmitted by contaminated individuals, the environmental stability and spread of norovirus have been well documented [Reference Lopman6, Reference Barclay25]. In fact, norovirus particles have been detected on environmental surfaces up to two weeks after initial contamination. Moreover, few household disinfectants have demonstrated efficacy in eliminating norovirus from contaminated surfaces [Reference Barclay25]. As such, the environmental hardiness of norovirus extends the potential for additional secondary household transmission despite the end of shedding and the best efforts of the household to eliminate the virus from common surfaces. Therefore, education on appropriate and effective procedures for cleaning and disinfecting household surfaces is essential to curbing the spread and environmental persistence of norovirus.
Interestingly, none of the outbreaks in this study were caused by norovirus GII.4, though it is the most common norovirus genotype causing outbreaks in the USA and worldwide [Reference Vega26, Reference Verhoef27]. This is important because innate susceptibility of humans to different norovirus genotypes, like GII.4, depends on the presence or absence of certain histo-blood group antigens [Reference Currier28]. Although the relationship between GII.4 and genetic susceptibility has been most clearly demonstrated, additional studies have also identified a relationship between the genotypes in this study (i.e., GI.6, GII.6, GII.12) and susceptibility to norovirus infection [Reference Currier28–Reference Prystajecky30]. For example, GII.12 has been shown to have a higher affinity to saliva samples from individuals with B and AB blood types than A and O, and a study among paediatric cases found 95% of GII.6 norovirus cases to be secretor positive [Reference Currier28, Reference Jin29]. Conversely, GI.6 infection was detected among a family with the G428A nonsense mutation of the FUT2 gene (i.e., secretor negative), which may indicate GI.6 infection susceptibility does not depend solely on the secretor status [Reference Prystajecky30]. Thus, all household contacts in this study may not have been equally susceptible to infection with the particular norovirus genotype in these outbreaks. In this study, it was not possible to assess host genetic susceptibility of norovirus among primary cases and their household contacts. Nonetheless, since the genetic composition of family members within the same household is likely similar, selection of families susceptible to norovirus may have occurred a priori through case ascertainment in the initial outbreak investigations.
Although this study advances the current understanding of the secondary household transmission of norovirus, there are certain limitations that should be considered. First, samples were not routinely collected and laboratory confirmation of norovirus infection was not possible for all cases. However, individuals from each outbreak had laboratory-confirmed norovirus infections, and the median period between presumed exposure and illness was generally consistent with norovirus infection in each of the outbreaks. Although most secondary cases fell within the normal incubation period for norovirus, some documented cases had unusually short or long incubation periods. This observation may be due to the potential misclassification of primary or secondary cases or ill individuals may not have had norovirus. Additionally, a lack of sample testing also prevented linking household secondary cases to primary cases through norovirus genome sequencing. Though laboratory confirmation of links between household cases was not possible, the timing of illness onset was consistent with previously reported secondary transmission of norovirus that did include laboratory linking of cases [Reference Gastanaduy15]. Second, not all individuals associated with the outbreaks could be contacted for follow-up, and it is possible that individuals included in this study may have had different risk factors from those who could not be assessed. However, there were no significant demographic and clinical differences observed between the primary cases interviewed and those who were not. Finally, some potential risk factors were not assessed and would merit further study, such as the physical size of the house, the number of bathrooms, the ratio of household members to bathrooms, the ratio of household members to bedrooms, food handling of primary cases, and any control measures implemented (i.e., household cleaning, case isolation, or improved hand hygiene).
In summary, this study identifies risk factors for secondary household transmission of norovirus and the degree to which they can extend the duration and scale of norovirus outbreaks. The number of primary cases and their symptoms were found to be the major risk factors in norovirus household transmission. Therefore, implementation of basic infection control practices, such as isolation of ill household members and enhancement of hand and environmental hygiene measures, may lead to a reduction in viral transmission. Further, the development of a norovirus vaccine capable of reducing viral shedding or mitigating the severity of symptoms would likely help to reduce household transmission. Future analyses should attempt to incorporate sample testing and genotyping of symptomatic and asymptomatic individuals, identification of individual secretor status, and more detailed information on symptom timing and severity, household characteristics, and implemented control measures.
ACKNOWLEDGEMENTS
We would like to acknowledge the Emory University Rollins School of Public Health Student Outbreak Response Team (SORT) for their assistance with conducting telephone interviews. We also thank the Wake County Health Department and North Carolina Division of Public Health for their contributions to the investigation of Outbreak 1, the Ottawa County Health Department for their contributions to the investigation of Outbreak 2, and the Wayne County Department of Health and Human Services for their contributions to the investigation of Outbreak 3. Finally, this study was supported in part by appointment to the Research Participation Program at the CDC (to ZM) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
None.






