INTRODUCTION
According to the World Health Organization (WHO), tuberculosis (TB) is the second most common cause of death from infectious diseases after HIV. In 2011, an estimated 1·4 million people died from the disease and 8·7 million new cases were reported [1]. Humans are not the only ones at risk of contracting TB. Elephants, predominantly the Asian species Elephas maximus, are known to be particularly susceptible to Mycobacterium tuberculosis and to M. bovis infections [Reference Michalak2–Reference Obanda10]. TB has been diagnosed worldwide in captive elephant populations [Reference Mikota3–Reference Mikota and Maslow6, Reference Mikota, Larsen and Montali9, Reference Lewerin11–Reference Verma-Kumar18], and a wild African elephant with previous human contact recently died from the disease [Reference Obanda10]. Palaeontologists have also hypothesized that TB may have played a role in the extinction of mastodons (Mammut americanum) since 52% of skeletons ever found showed typical TB lesions [Reference Rothschild and Laub19]. The transmission of M. tuberculosis between elephants and humans was documented for the first time in 1998 in an exotic animal farm that housed four infected elephants in Illinois, USA. Half of the 22 animal handlers had positive tuberculin skin tests and one was diagnosed with active TB. Molecular analyses showed that isolates from the elephants and the infected handler were identical [Reference Payeur5]. Several reports have since confirmed that caretakers who have close and prolonged contact with elephants shedding tubercle bacilli were at risk of being infected through aerosols [Reference Michalak2, Reference Montali, Mikota and Cheng4, Reference Murphree12, Reference Granich Oh20–Reference Stephens22]. A recent study in Malaysia using the QuantiFERON assay (Cellestis Limited, Australia) showed that elephant handlers had much higher TB prevalence than the general population (25% vs. 4–6%) [Reference Ong17]. Four cases of culture-positive Asian elephants with strains of human origin were also recently reported in Thailand [Reference Angkawanish14].
Lao PDR (Laos) is one of the last countries where Asian elephants are still used in the timber industry. There are about 500 at work in logging camps and as many in the wild [Lao Elephant Care and Management Programme (LECMP), unpublished data]. The ownership of an elephant can be shared between several people, and some owners may employ more than one mahout to ride their elephants. As a result, there are often two or more persons working every day in close contact with each animal. According to the WHO numbers, Laos was ranked second in terms of human TB prevalence among the 13 Asian elephant countries in 2011 [1]. TB prevalence, incidence and mortality were estimated to be 540, 213 and 11/100 000 inhabitants, respectively [23]. Given the proximity between mahouts and elephants, there is a concern that transmission of M. tuberculosis from both parties may be frequent. Because contacts between captive and wild elephants are common in several areas of Laos, the risk of spreading TB to wild populations could also be significant [Reference Duffillot, Maurer and Bouchard24].
The prevalence of TB in animals has never been studied in Laos and literature on human TB is scarce. The purpose of this study was thus to estimate the prevalence of the disease in domestic elephants and their mahout in Laos, in order to determine whether exposure to elephants is a major risk factor of being infected.
METHODS
Study design
A cross-sectional study was conducted from January to July 2012 in the four districts surrounding the Nam Pouy National Park in the province of Sayaboury, Laos: Thongmixay, Paklay, Phieng and Sayaboury (Fig. 1). This region hosts more than 80% of the domestic elephants and is home to the second largest population of wild elephants in the country, estimated to be 60–80 animals (LECMP, unpublished data). In each district, the elephant owners were located and contacted by the local District Agriculture and Forestry Office. Based on this information, a field survey was organized to examine the elephants, the mahouts and the owners either in their village, in logging camps, or at the annual elephant festival in the town of Sayaboury. Data was collected by a team of veterinarians and medical doctors coordinated by the LECMP. Written informed consent was obtained from all recruited participants and ethical approval was granted by the Lao National Ethics Committee for Health Research.

Fig. 1. Sayaboury province, Lao PDR.
Recruitment and TB screening
Elephants
Elephants aged ⩾10 years were included in the study group. Data on health status and on previous contacts with humans and other elephants (wild or domesticated) were collected from owners and mahouts. The veterinary team performed a clinical examination of the animal and collected 5 ml of whole blood. Fresh serum samples were aliquoted, transported on ice to the National Animal Health Centre in the capital city of Vientiane and stored at −80 °C. Samples were then tested with the ElephantTB Stat-Pak test (Chembio Diagnostic Systems Inc., USA), based on serological detection of IgM and IgG antibodies specific for M. tuberculosis and M. bovis. The rapid tests were performed and interpreted according to the manufacturer's instructions and read by two operators. Trunk washes could not be performed in the field because of difficulties in implementing the method on untrained elephants.
Mahouts and elephant owners
Every person aged >15 years owning and/or working with an elephant for >1 month was included in the study. Socioeconomic and medical data were recorded as well as frequency and duration of contact with elephants. Comprehensive clinical examinations were then conducted by our medical team. Chest X-rays were performed at the district hospital by the local radiologist and double checked by a pneumologist at the referral hospital of Mahosot in Vientiane. Two sputum samples were collected from each participant, the first at the time of consultation and the second in the morning upon waking [Reference Parsons25]. Salivary samples or sputum specimens of insufficient volume were discarded. Microscopic examination of Ziehl–Neelsen-stained smears [26] was performed by a laboratory technician from the district hospital. At least 200 microscopic fields were read at 1000× magnification before asserting the absence of acid-fast bacilli. Aliquots of sputum samples were also transported on ice by plane or bus within 72 h to the National TB Reference Laboratory at Mahosot Hospital for a second independent smear microscopy and for Mycobacterium culture. Briefly, after homogenization and decontamination for 15 min with an equal volume of sterile 4% sodium hydroxide solution, three drops of specimen were inoculated on slopes of solid Ogawa medium [Reference Kudoh and Kudoh27]. Cultures were incubated at 36 °C for 8 weeks and tubes with contaminants were discarded. Culture-positive tubes were sent for Mycobacterium species identification and for susceptibility testing at the Centre d'Infectiologie Christophe Mérieux du Laos, Vientiane, using the GenoType MTBDRplus and GenoType Mycobacterium CM/AS assays (Hain Lifescience GmbH, Germany) according to the manufacturer's instructions.
Statistical analysis
Data entry was performed using EpiData v. 3.1 software (EpiData Association, Denmark) and analysis with Stata v. 8.0 (Stata Corp LP, USA). Qualitative variables were compared with χ 2 or Fisher's tests. Quantitative variables with normal distribution were compared with Student's t test and ANOVA tests while Wilcoxon and Kruskal–Wallis tests were used for the comparison of quantitative variables with abnormal distribution. A 0·05 level of significance was used for the analyses.
RESULTS
Elephants
Study population
A total of 82 elephants, representing 23% of the domestic population of the province of Sayaboury and 18% of the national population (LECMP, unpublished data), were examined by the veterinary team. Two elephants were too aggressive to be approached and were excluded from the study. The remaining were aged 13–59 years (mean 33 years) with a F/M sex ratio of 1·58/1 (Table 1). Twenty (25%) were from Paklay district, 19 (24%) from Thongmixay, 14 (18%) from Phieng and 10 (13%) from Sayaboury. The remaining 17 elephants were working in Phieng during the study, but came from nearby districts: 15 (19%) were from Hongsa and two (3%) were from Botene. All the animals were used for timber extraction. The majority (84%) were living with or had regular contacts with other working elephants while 25 (31%) had regular contacts with wild elephants. Of those with regular contacts with wild animals, 22 (92%) were females that had been approached by wild males when kept in the forest near their logging camps or villages, during the breeding season.
Table 1. ElephantTB Stat-Pak assay results according to different epidemiological variables
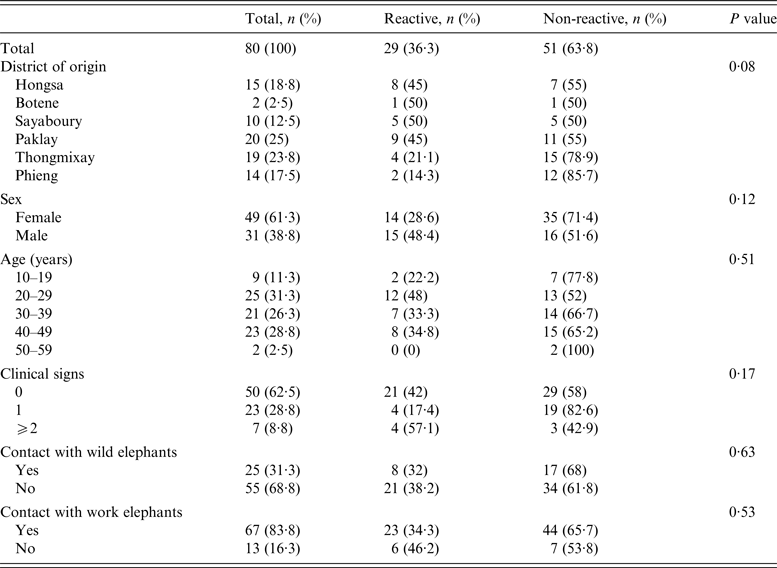
Clinical findings
When the veterinary team visited, 19/80 elephants (24%) had abscesses, wounds or injuries, mainly on the shoulders, head or back. Six females were lactating and six others were thought to be pregnant. Clinical signs suggestive of TB in elephants [Reference Mikota3, Reference Mikota and Maslow6, Reference Angkawanish14, Reference Landolfi15] were investigated: fatigue, weight loss, loss of appetite and trunk discharge. Of the 80 screened elephants, 50 (62%) showed no clinical signs, 23 (29%) showed one sign and seven (9%) showed two or more (Table 1). Interviews revealed that 13 (16%) elephants had trunk discharge when working hard in hot weather.
ElephantTB Stat-Pak reactivity
Of the 80 elephants tested, 29 (36%) were reactive to the ElephantTB Stat-Pak assay (Table 1). Males were more frequently reactive (48%) than females (29%), although the difference was not significant (P = 0·07). Reactivity differed with the district of origin and was higher in Hongsa (53%), Sayaboury (50%) and Paklay (45%) while lower in Thongmixay (21%) and Phieng (14%). However, this difference did not appear significant (P = 0·08). Reactivity of the elephants did not significantly correlate with clinical signs suggestive of TB (P = 0·17) or with age (P = 0·51) and sex (P = 0·17).
Elephant owners and mahouts
Study population
In total, 142 participants (after exclusion of the two mahouts of the two aggressive elephants) were enrolled in the study, with 1–4 individuals (mean 1·8) per elephant. All were men, aged between 16 and 68 years (mean 35·7 years), mostly married (77%) and belonging to the Lao Loum ethnic group (92%), the predominant ethnic group in Laos. Only 40% had received the bacille Calmette-Guérin (BCG) vaccine. Most participants were mahouts (48%), 30% were only owners (some owned several elephants) and 23% were both mahouts and owners. They had spent an average of 17 years with work elephants (range 1–50 years) and an average of 10 years with the elephant included in this study (range 1–50 years). Of the participants, 35% owned or worked with an elephant reactive by the ElephantTB Stat-Pak assay.
Clinical findings
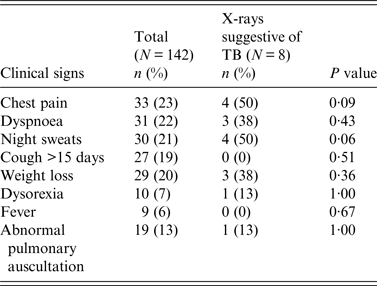
Upon examination, 90 (63%) participants had at least one clinical sign suggestive of TB [28]: chest pain (23%), dyspnoea (22%), night sweats (21%), cough for >15 days (19%), weight loss (20%), crackling or wheezing during pulmonary auscultation (13%), dysorexia (7%) and fever (6%) (Table 2). Thirty-one (22%) showed only one suggestive sign, 28 (20%) two signs and 30 (21%) ⩾3 signs. Although 55% of the participants declared smoking cigarettes, this was not significantly associated with any clinical signs suggestive of TB or with abnormal chest X-rays (P = 0·77).
Table 2. Clinical and radiological signs suggestive of tuberculosis among mahouts and elephant owners
Chest radiography
Eight (6%) participants had chest X-rays suggestive of pulmonary TB (Table 3). Three of these (nos. 86, 99, 104) worked with a Stat-Pak-reactive elephant and seven had familial or personal history of TB. Six of these eight participants also had clinical signs suggestive of TB. They were all seen 3 months later; clinical examinations were then normal and Ziehl–Neelsen stain and culture gave negative results.
Table 3. Demographic and clinical features of participants with medical history of TB, abnormal X-rays or with positive mycobacterial culture
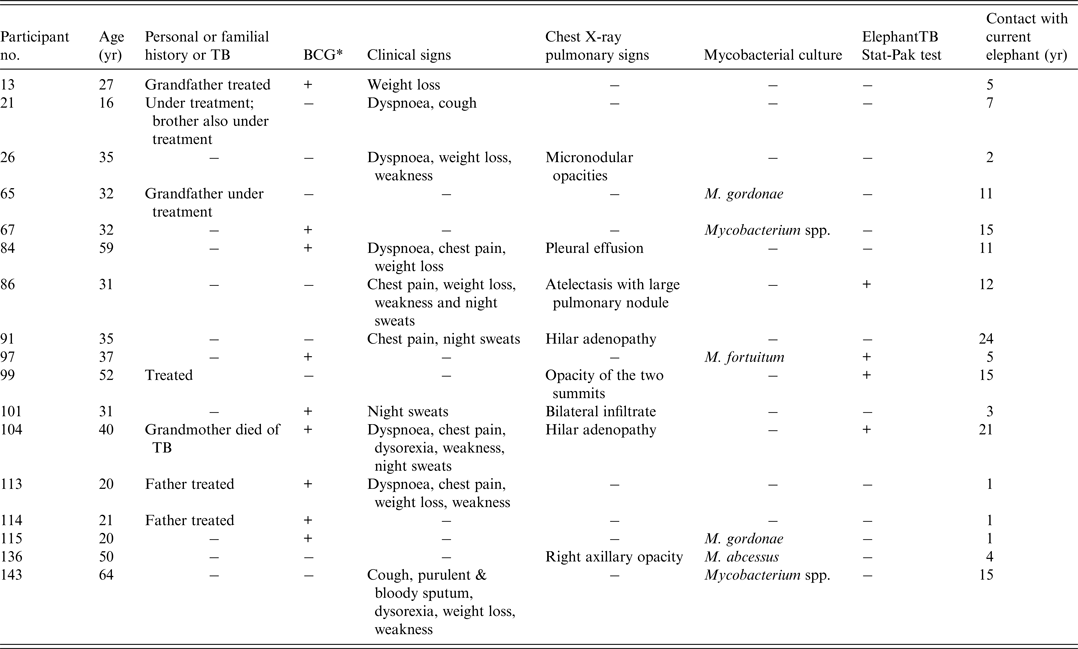
* Previous bacille Calmette-Guérin (BCG) vaccination.
Culture and microscopy
Two sputum samples per participant were collected for a total of 286 samples, of which 164 were suitable for analysis. All samples were negative for acid-fast bacilli. Upon culturing, 19 (8%) were discarded because of contamination. Seven samples from six different participants were positive for Mycobacterium species. All these mycobacteria were identified as Mycobacteria other than tuberculosis (MOTT): M. gordonae (n = 2), M. fortuitum (n = 1), M. abscessus (n = 1) and unidentified species (n = 2). Some of these MOTTs were isolated from participants with clinical or X-ray signs of TB (nos. 136 and 143), with TB-infected relatives (no. 65), or with a Stat-Pak-reactive elephant (no. 97) (Table 3).
Data analysis revealed no significant relationship between culture, clinical or radiographic signs suggestive of TB and elephant reactivity with the ElephantTB Stat-Pak test.
DISCUSSION
The aim of this survey was to report the seroreactivity of TB in working elephants in Laos and the TB-related health status of their mahouts and owners. A total of 80 elephants and 142 mahouts/owners were examined either in their village, in logging camps, or at the annual elephant festival in the town of Sayaboury. This represented more than 18% of the total national population of registered working elephants (LECMP, unpublished data). The seroreactivity in the elephant population found in this study appears surprisingly higher (36%) than in the other Asian countries where the ElephantTB Stat-Pak assay has been used before. In Nepal and Malaysia, TB seroreactivity was found to be 15% (n = 106) and 20% (n = 63), respectively [Reference Landolfi15, Reference Ong17]. In India, a recent study on 179 serum samples randomly selected from 600 working elephants showed a seroreactivity of 18% [Reference Verma-Kumar18]. Higher rates were found in temple elephants (25%) compared to other groups of working elephants (12–15%) [Reference Abraham16]. These findings suggest that close contacts between humans and elephants in countries with high TB may increase the risk of TB infection in elephants. The higher elephant seroreactivity observed in our study could reflect the high prevalence of human TB in the country. In Laos, the TB burden has been found to be 540/100 000 whereas in Malaysia it is 101/100 000, in Nepal 243/100 000 and in India 249/100 000 [1, 23]. The recent increase in elephant resale in Laos could also contribute to the high seroreactivity. National data show that about 50% of working elephants have changed owners in the last decade (LECMP, unpublished data). This implies increased contacts between elephants and sequential owners, mahouts and their community.
Our results show that elephant seroreactivity was significantly influenced by the district of residence (from 14% to 53%). This relationship has already been reported in other studies, notably in Malaysia where seroreactivity ranged from 0% to 26% in herds from different locations. However, groups there were too small to highlight the factors explaining these differences [Reference Ong17]. In our study, the lowest rates were found in the districts of Thongmixay (21%) and Phieng (14%). In those districts mahouts traditionally work around the Nam Phouy National Park where logging is intensive; therefore, they are thus less likely to travel long distances to reach new logging camps. Isolation from other elephants and mahouts could be a potential protective factor against TB exposure in working elephants.
Our results also suggest a greater susceptibility of male elephants to TB infections (48% vs. 29% for females, P = 0·07). To our knowledge, such a difference has never been reported before. In Nepal, no association was found between sex or age and reactivity to the ElephantTB Stat-Pak assay [Reference Landolfi15]. Similarly, US data from 1994 to 2011 showed that the incidence of TB culture-positive elephants was 11% in both males and females [Reference Orloski8]. In the logging camps of Laos, male elephants are usually given higher workloads than females in order to avoid aggressive behaviours, particularly during the musth period. A chronic state of fatigue in males could result in an impairment of their immune system and explain their greater susceptibility to TB infection, as suggested for humans [Reference Chrousos29]. Further studies would be needed to confirm such claim.
Around a third (31%) of the elephants included in this study had contacts with wild elephants (Table 1), exclusively inside or around the Nam Phouy National Park. Most of them (88%) were domesticated females temporarily released into the forest during the breeding season to be approached by wild tuskers. This traditional method of elephant breeding is by far the most successful in Laos and remains crucial for the survival of the population. In 2011, when at least 17 deaths were recorded, 5/6 newly born elephants in the whole country had a wild father (LECMP, unpublished data). The high TB seroreactivity in working elephants suggests that the wild breeders could be infected. It is likely that these males also breed with the wild females that are known to live in the park in three or four matriarchal groups comprising a total of 60–80 individuals (K. Khounboline, personal communication), and hence transmit mycobacteria to the wild population. Little is known about the consequences of the introduction of TB in a wild Asian elephant population, especially its effects on reproduction. Later stages of the disease are, however, known to markedly affect the general health condition of the animals [Reference Payeur5]. This could hasten the extinction of small and isolated populations of elephants like those living in the Nam Phouy National Park.
Despite high TB seroreactivity in elephants, neither mahouts nor owners were smear- or culture-positive for M. tuberculosis or M. bovis although MOTTs were isolated from six (4%) participants and although eight (6%) participants had chest X-rays suggestive of TB. These atypical mycobacteria have been isolated from 15% of smear-positive participants in a recent TB prevalence survey in Laos (National Tuberculosis Programme, unpublished data). Infections by MOTTs are not considered contagious for humans or other animals [Reference Griffith30] but can be pathogenic and are probably underdiagnosed in regions where TB is endemic, e.g. in Southeast Asia [Reference Phillips, Gopinath and Singh31, Reference Simons32]. M. gordonae, which was cultured from the sputum samples of two participants is generally found in contaminated water networks but is considered non-pathogenic [Reference Dailloux and Blech33].
The discrepancy between the high seroreactivity in elephants and the absence of active pulmonary TB in their mahouts or owners could partly be explained by the tests used in this study. The ElephantTB Stat-Pak assay is a screening test that detects both IgG and IgM. Anti-TB IgG may be detected long before the appearance of clinical signs and may persist for years, even after treatment of the elephants [Reference Lyashchenko34]. False-positive results have been known to occur with the ElephantTB Stat-Pak; some have been attributed to MOTT infections [Reference Lacasse7] or to chronic inflammatory diseases such as arthritis [Reference Lyashchenko34–36]. In our study, 7/29 reactive elephants (24%) presented with inflammation (cutaneous abscesses, wounds or corneal perforations). This could have impacted their reactivity status. It has also been suggested that an anergic immune response in an advanced TB case could interfere with the ElephantTB Stat-Pak assay [Reference Angkawanish14]. Clinical manifestations of TB in elephants are usually observed in later stages of the disease. This may explain why no correlation was found between seroreactivity and clinical signs in this study, or in others [Reference Angkawanish14, Reference Lyashchenko37]. Further tests should indeed be performed in order to confirm the Stat-Pak-reactive results. It is generally assumed that the response to TB by the immune system is cell-mediated. However, the tuberculin skin test is not applicable in elephants and the development of an interferon gamma release assay (IGRA) is currently underway in Thailand [Reference Angkawanish38]. Several studies have found that serodiagnosis in elephants is more accurate than in other species. Many African as well as Asian elephants did produce strong antibody responses years before mycobacteria were isoled by trunk wash [Reference Lyashchenko34]. Serological tests have therefore been used as well as trunk-wash cultures. Although culture remains the most reliable technique to confirm active TB in elephants, it has inherent limitations as screening diagnostic technique. Several weeks of incubation are needed before results can be obtained, it requires prior training of the elephants and has limited sensitivity [Reference Mikota and Maslow39]. As well as the Elephant Stat-Pak test, other assays have been developed. However, multi-antigen print immunoassay (MAPIA), which allows the detection of a broad range of antibodies, must be performed in a laboratory-based setting and is more useful for post-treatment follow-up [Reference Lyashchenko37, Reference Lyashchenko40]. The Dual Path Platform assay (DPP Vet TB test; Chembio Diagnostic Systems Inc.), that allows the discrimination between MOTTs and M. tuberculosis complex [Reference Greenwald35, Reference Lyashchenko37], was unfortunately not available when this study was conducted.
In this study mahouts and owners were considered TB-positive if sputum samples turned culture-positive. Only active pulmonary TB would therefore have been diagnosed here, but this could have been underestimated; although the combination of sputum microscopy and culture is extremely specific and is considered the gold standard, the sensitivity has been reported to be only 78% [Reference Levy41]. Sensitivity of the Ziehl–Neelsen stain on its own has been shown to vary greatly [Reference Steingart, Ramsay and Pai42] and chest X-rays might not reveal suggestive signs of TB in the early stages of the disease, when damage to the lungs have not yet become apparent. Numbers of TB-infected participants might thus have been underestimated in our study.
The absence of detection of active pulmonary TB cases in the 142 participants suggests that mahouts and owners do not show a higher risk of disease than the general population of Laos. The country has the second highest TB burden of the 13 Asian elephant countries after Cambodia [23]. In this context, it is likely that the transmission of TB occurs from humans to elephants. A medical monitoring of people working or living with elephants should thus be implemented.
ACKNOWLEDGEMENTS
We thank the Fondation du XXIIIe Congrès Mondial Vétérinaire and the ElefantAsia NGO for their financial support, as well as Elephant Care International for providing the ElephantTB Stat-Pak assays. We also thank all the mahouts and elephant owners who kindly participated in this study, and the Elefant-Asia NGO and its staff, especially Gilles Maurer, Thongsavath Douangdy, Mylene Bastien-Larochelle and Xaileng Kaokeu for their assistance during field work. We gratefully aknowledge the collaboration of the Lao Ministry of Agriculture and Forestry and its District Agriculture and Forestry Officers, the Lao Ministry of Health, the Sayaboury and Paklay district hospitals, Irwin Law and the National TB Reference Laboratory at Mahosot Hospital, the Centre d'Infectiologie Christophe Mérieux du Laos, and Guy Beauchamp from Faculté de médecine vétérinaire de l'université de Montréal for helping with statistical analyses.
DECLARATION OF INTEREST
None.







