INTRODUCTION
Tuberculosis (TB) caused by infection with Mycobacterium tuberculosis remains a major global health problem with an estimated 8·8 million cases worldwide in 2011 [1]. Australia is a low-incidence country for TB, with annual notification rates between 5 and 6/100 000 population. Most cases diagnosed in Australia are migrants from high-incidence countries and local transmission events are uncommon [Reference Barry2, Reference Roberts-Witteveen3]. In New South Wales (NSW), a state of Australia, TB is notifiable under the NSW Public Health Act 2010 and a database of notifiable diseases is maintained by the NSW Ministry of Health (MoH) [Reference Roberts-Witteveen3].
M. tuberculosis, a highly clonal pathogen, is considered to be primarily a pathogen of humans and was a cause of human infections long before the evolution of animal-adapted lineages [Reference Smith4]. With the close association between humans and animals, transmission can occur. Animals generally acquire TB infection from humans. Animal-to-animal transmission can occur, including between different species [Reference Isaza, Fowler and Miller5, Reference Montali, Mikota and Cheng6], and animals can also be a potential source of infection for humans. In the majority of cases, infection is acquired following close and/or prolonged contact with an infected animal through occupational exposure [Reference Montali, Mikota and Cheng6–Reference Posthaus8, Reference Davis9–Reference Une and Mori13].
In 2006, an Australian zoo imported five Asian elephants (Elephas maximus) from Thailand. They were screened for TB during pre-export quarantine and then annually after arrival at the zoo, using a triple trunk wash method (TW) [14].
In April 2009, the zoo commenced using a new TB antibody test for elephants, the ElephantTB Stat-Pak® (SP; Chembrio Diagnostic Systems Inc., USA) and in February 2010 the Dual Path Platform Vet®TB test™ (DPP; Chembrio Diagnostic Systems Inc.) [Reference Greenwald15]. One elephant was strongly reactive on both tests. Retrospective testing of serum from the elephant from Thailand (2004) and after arrival in Australia (2006) were reactive on the antibody tests, indicating the elephant was infected prior to importation. Trunk-wash samples remained negative for this clinically healthy elephant until a single trunk-wash sample taken 3 weeks after parturition in November 2010 was culture-positive for M. tuberculosis. Although previous shedding could not be ruled out, it was thought that the birth resulted in reactivation of a latent infection. The elephant was therefore potentially shedding for 44 days without knowledge (parturition to culture confirmation).
Treatment of the infected elephant with isoniazid, rifampicin and pyrazinamide commenced immediately. Monthly TW and DPP screening commenced. The elephant's isolate was fully susceptible to all first-line antituberculous agents. The elephant remained on three drugs for 1 month and completed a 12-month course of isoniazid and pyrazinamide. The isolate was genotyped as a Beijing strain, which is predominant in China and is common in South East Asia. It accounts for about 37–44% of human cases in Thailand, where isolates have been typed [Reference Van Soolingen16, Reference Prodinger17].
In November 2009, the zoo's chimpanzees (Pan troglodytes) were moved to a temporary facility while their exhibit was being refurbished. All chimpanzees tested negative for TB [using a comparative tuberculin skin test (TST) and interferon-γ release assay (IGRA)] (Primagam, Prionics, Switzerland) during routine health checks undertaken at the time of their move. In April 2011, one male chimpanzee was noted to have lost weight, be lacking energy, and to have developed an intermittently discharging groin wound. In September 2011, the chimpanzees were anaesthetized for their move back to their refurbished facility. Examination of the sick male chimpanzee revealed poor body condition, weight loss and multiple enlarged lymph nodes. Pus draining from his groin wound contained acid-fast organisms. A TB antibody test (SP and DPP) was reactive, IGRA was positive, and chest radiographs showed significant pathology in one lung. Due to the severity of his illness, the chimpanzee was euthanased. Necropsy showed severe disseminated TB disease confirmed by PCR and culture.
Mycobacterial interspersed repetitive unit (MIRU) typing revealed that the genotypes of M. tuberculosis recovered from the elephant (MIRU-12 = 223 325 173 532; MIRU-24 = 144 644 423 242) and the chimpanzee (MIRU-12 = 223 325 173 532; MIRU-24 = 14 474 4423 242) had the same number of tandem repeats in 23 loci out of 24 and one tandem repeat difference in QUB-116 locus, suggesting a matched strain of disease [Reference Malakmadze18].
This paper describes the epidemiological investigation following identification of the two linked cases of M. tuberculosis infection in the zoo. This study aimed to identify infection in people exposed to the animals, to understand the risk factors for infection, and to determine how the probable transmission from the elephant to the chimpanzee occurred.
METHODS
The study is described in two phases: phase 1, conducted in February 2011, which aimed to identify persons and animals at risk of infection following diagnosis of M. tuberculosis in the elephant; and phase 2, commenced in September 2011, which was a broader study following diagnosis of M. tuberculosis in the chimpanzee.
This research was undertaken in accordance with local public health legislation, thus human research ethics approval was not necessary.
Extent of infection in animals
Following diagnosis of M. tuberculosis in the elephant, other mammal species within the immediate vicinity (20 m) of the elephant enclosure were screened for TB. The elephant's calf was considered at high risk of becoming infected. As soon as she was trained, monthly TW and DPP screening commenced. TW and SP screening for all other elephants was increased to every 3 months [14].
Following the chimpanzee's diagnosis, all other chimpanzees were screened for TB during their move, and screening of other mammals in the zoo was expanded. Animals were prioritized for screening based on proximity to the elephant and chimpanzee, susceptibility to TB, the availability of tests recommended for screening for TB in particular species, and ease of testing. All outgoing mammal transactions ceased to avoid potential transmission to other zoological institutions until the extent of infection could be established.
The infectious period for the elephant was defined as August 2010 to February 2011, inclusive, and the infectious period for the chimpanzee was defined as January 2011 to September 2011, inclusive.
Extent of infection in humans
Enhanced infection control measures and precautions were implemented, including extension of viewing barriers around the elephant enclosure and cessation of any close visitor contact.
In phase 1, an assessment of work practices at the zoo was undertaken to identify staff that were at risk of exposure to the elephant during its infectious period. These staff, and other staff who expressed concern about potential exposure (but not classified as being exposed to the elephant), were screened by TST.
During phase 2, the study population was widened. Staff working within an area of extended concentric circles around both animals, staff working within a 10-m zone outside the two animal enclosures, and staff working within the area between the two animals were defined as our human study population. The perimeter around the elephant included a pedestrian area at the back of the elephant barn, classified as part of the elephant enclosure and inaccessible to the public, that housed a refrigerated room used for storing browse (leaves and branches of shrubs or trees used as animal food). A large unsealed door separated the elephant barn from this area, with gaps large enough for the elephants to protrude their trunks at the sides and bottom of the door. The infected elephant and her calf had access to the area directly behind this door. Browse was regularly placed on the concrete in the pedestrian area in front of the refrigerator for sorting and distribution to other areas of the zoo. Although the area was kept clean, there was potential for the browse to come into contact with elephant faeces and discharges.
Staff not screened by TST during phase 1, were now screened by TST. All study participants were interviewed with a standardized questionnaire designed to clarify activities and time spent in areas of the zoo where any exposure to the elephant and/or chimpanzee may have occurred. Demographic, clinical (including previous infection with TB), country of birth, bacille Calmette-Guérin (BCG) vaccination status, and travel and residency history data was also collected.
High-level contact with the elephant was defined as at least 5 h of direct physical contact with elephants during the infected elephant's infectious period. This included activities such as providing healthcare, feeding, grooming or training the elephants.
The prevalence of TB infection that may be attributable to this event was defined in three ways, with increasing specificity but decreasing sensitivity:
(1) Positive TST: that is, in all people who had a TST during the post-exposure survey, the proportion who had TST ⩾10 mm.
(2) Positive TST in people without other risk factors: that is, in people who had a TST during the post-exposure survey, who did not have BCG scar and who had not had prolonged residence in a high burden country, the proportion who had TST ⩾10 mm.
(3) TST conversion: that is, in people whose last TST prior to the elephant's infectious period was recorded as negative and who had a TST performed during the post-exposure survey, the proportion who had an increase in TST size ⩾10 mm compared to their pre-exposure TST.
Mechanisms of transmission studies
Various hypotheses for transmission were explored:
Animal intermediate
Any wild mammals found dead or that required euthanasia in the zoo grounds were screened for TB at necropsy. In addition, a retrospective study of 33 common brushtail possums (Trichosurus vulpecula) and 33 common ringtail possums (Pseudocheirus peregrines) from zoo grounds was conducted using archived material (fixed tissue in wax blocks, recut and stained for acid-fast bacilli).
Human intermediate
A search was undertaken of the database held by the MoH to determine whether any cases of M. tuberculosis had been notified in people employed at the zoo. MIRU-type profiles (24 digits) [Reference van Deutekom19] were conducted on all human isolates collected in NSW from 2006 (when the elephant arrived in Australia) to June 2011. Other Australian jurisdictions were contacted to collect information on results of genotyping to determine any matches with the elephant and chimpanzee. MIRU profiles for all available human isolates were compared with the MIRU profiles of the elephant and chimpanzee cases.
Fomite transmission
Fomites such as browse, tools, veterinary equipment, clothing, waste disposal bins and faeces were considered as potential mechanisms of transmission. A detailed review of husbandry, cleaning and sanitation practices, browse distribution, staff movements, waste disposal bin and vehicle movements and sharing of tools between elephant and chimpanzee facilities, and elephant faeces disposal pathways and methods was undertaken. Veterinary and animal-keeping records were examined to determine if equipment used on the elephants might have subsequently been used on the chimpanzees.
Airborne transmission
On-site inspections, analysis of zoo practices and of prevailing winds were conducted to assess plausible airborne transmission between the elephant and the chimpanzee. Seasonal wind direction patterns were obtained from the Australian Bureau of Meteorology.
Vector
Consideration was given to the possibility of vector transmission. There was no method to test this mechanism of transmission.
Laboratory methods
All M. tuberculosis isolates were identified by high-performance liquid chromatography (HPLC) of mycolic acids (Waters™ LS Module 1Plus, USA) and DNA probes and in-house PCR, targeting the 16S-23S rRNA gene internal transcribed spacer region, when necessary [Reference Xiong20]. Isolates were genotyped by MIRU typing (24 digits) [Reference van Deutekom19]. Quality control strains of M. tuberculosis and M. bovis BCG were used to monitor the performance of genotyping techniques. Isolates were assigned to lineages defined in the online MIRU-VNTRplus database [21].
Statistical methods
We performed a univariate analysis, calculating the relative risks (RR) and 95% confidence intervals (CI) for each of the exposure variables. Fisher's exact test was used to determine statistical significance where the relative risk was incalculable. Variables which had a raised relative risk on univariate analysis as well as those which were potential biases of TST results (BCG vaccination or prolonged residence in a high burden country) were included in a multivariate logistic regression model. Statistical analyses were performed using SAS Enterprise Guide v. 5·1 (SAS Institute Inc., USA).
RESULTS
Extent of infection in animals
Blood was collected from the calf in August 2011. Both SP and DPP were strongly reactive indicating the presence of antibodies to M. tuberculosis. These antibodies may have been maternal, through placental transfer and/or colostrum, or fetal in origin. The finding that the intensity of the calf's DPP reaction waned over several months without treatment suggests that antibodies were probably maternal in origin.
TW results for all other elephants were negative. SP remained non-reactive for all apart from one other cow that seroconverted in December 2011. This animal was placed on a 9-month course of prophylactic treatment. No evidence of infection was found in any other animals screened in phase 1.
Screening of the other 17 chimpanzees at the time when the chimpanzee case was diagnosed identified six with suspected TB infection but none with TB disease. With the exception of one 60-year-old female chimpanzee that had no evidence of infection and was not treated due to advanced age, all chimpanzees were placed on prophylactic treatment (isoniazid and rifampicin or rifabutin for 9 months). Repeat TB screening (TST, IGRA, SP, chest radiographs, tracheal wash) and physical examination of all chimpanzees was undertaken in January/February 2012. There was no evidence that any animal had TB disease. Those animals with evidence of TB infection at the initial screening remained on treatment. No new infections were identified and treatment was stopped for all other chimpanzees.
At the time of reporting, 266 live collection mammals had been screened. Of these, 30 required follow-up screening due to suspicious results. A further 16 animals had repeat screening done opportunistically. Follow-up and repeat screening numbers do not include the elephants. One hundred and thirty-one dead collection mammals and 43 wild mammals euthanased or found dead in the zoo grounds were screened for TB at necropsy. A retrospective study of 38 Northern palm squirrels (Funambulus pennantii) was conducted using archived material (fixed tissue in wax blocks, recut and stained for acid-fast bacilli). No case of TB was diagnosed in any live or dead collection mammal or any wildlife from zoo grounds. Where there were suspicious results, screening was repeated, in some cases on numerous occasions. Where lesions, particularly in the lungs was suspected, advanced diagnostics modalities such as computed tomography, ultrasound examination and fine needle aspiration of suspected lesions were used to exclude a diagnosis of TB.
Extent of infection in humans
Phase 1 assessment identified 22 staff with a high level of contact with the infected elephant. In addition, 30 staff self-presented for screening. TST screening of these 52 staff identified three people with TST conversions. Two had high-level contact with the elephant, and one worked within the elephant enclosure during the elephant's infectious period. All staff with TB infection received clinical follow-up in accordance with MoH guidelines. Clinical assessment of infected staff by expert physicians did not identify any cases of TB disease.
In phase 2, no cases of TB disease were found in zoo staff. Exposure information was collected for 138 staff. Fifty-eight (42%) had TST screening prior to the elephant's diagnosis; 50 (36%) had previous negative and eight (6%) had previous positive TST results. Three (2%) staff members who had pre-exposure TST screening did not complete TST screening during the investigation. Nine (7%) staff in total declined any TST screening. One staff member, who during the elephant's infectious period had worked within the elephant enclosure, had a TST conversion identified during this phase 2 screening (Fig. 1).
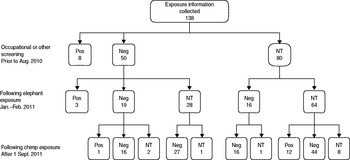
Fig. 1. Tuberculin skin test (TST) screening conducted prior to animal exposure, and during the two phases of the investigation, including results. Pos = TST reading ⩾10 mm diameter; Neg = TST reading <10 mm diameter; NT = not tested.
Altogether 16/118 (14%) staff members who had a TST after the elephant and/or chimpanzee's infectious period were positive and 7/67 (10%) staff members who did not have other risk factors for a positive TST and had a TST after the elephant and/or chimpanzee's infectious period were positive. There were four TST conversions in the 47 (9%) staff members who had pre- and post-exposure TST (Table 1).
Table 1 Association between single tuberculin skin test (TST) ⩾10 mm or TST conversion in humans and exposure to the elephants and chimpanzees

BCG, Bacille Calmette-Guérin; RR, relative risk; CI, confidence interval.
* Case: TST ⩾10 mm.
† ⩾3 months, cumulative.
‡ Case: increase in TST ⩾10 mm compared to previous TST performed prior to the elephant's infectious period.
§ Number of cases/number of subjects.
|| Where the relative risk was incalculable, Fisher's exact test was used to calculate statistical significance. In both cases P > 0·1.
¶ Defined as having entered the area inside the enclosure for any time during the animal's infectious period.
# High-level contact defined as at least 5 h of direct physical contact with the elephants during the infected elephant's infectious period.
There were no significant predictors for a single positive TST when all subjects were included in the analysis. When subjects with other risk factors for a positive TST were excluded, the association between exposures to the elephant and having a positive TST increased but were not significant (Table 1).
All four cases with TST conversions had spent at least 10 h (range 14–1064 h, median 584 h) within the elephant enclosure during the elephant's infectious period. High-level contact with the elephant was the only significant predictor of TST conversion based on univariate analysis (RR 6·8, 95% CI 1·2–39·8). Raised relative risks were found with maintenance and/or cleaning the elephant exhibit (RR 7·1, 95% CI 0·8–62·3), sorting browse behind the elephant barn (RR 7·1, 95% CI 0·8–62·3) and handling browse inside the elephant exhibit (RR 2·9, 95% CI 0·5–18·5). No other variables were associated with TST conversion status (Table 1).
Analysis of data by multivariate logistic regression determined that no single exposure independently predicted either TST conversion or a single TST ⩾10 mm.
Transmission pathways
Opportunistic and retrospective screening of potential animal intermediates found no evidence of M. tuberculosis. No human cases of TB on the MoH database were epidemiologically linked to the disease in the animals. No human isolates in the NSW MIRU database or reported from other jurisdictions in Australia matched the genotype found in the animals.
No equipment or staff were common to both elephants and chimpanzees or to their exhibits. Surplus browse was occasionally transferred by the primate keepers from the storage and sorting area behind the elephant barn to the chimpanzee exhibit. The main supply of browse to the chimpanzees was from another area of the zoo. Primate and elephant keepers and other staff shared the pedestrian area behind the elephant barn. Occasionally elephant waste bins containing faeces were moved through this area and occasionally the elephants were walked through this area. Elephant faeces were transported in a separate vehicle and sent for composting. Waste bins were cleaned and disinfected once emptied, prior to re-distribution. There were no veterinary procedures where equipment used for the elephants was subsequently used on the chimpanzees.
The distance between the elephant exhibit and temporary chimpanzee enclosure was 110 m. There were numerous large buildings and vegetation including tall trees between the two areas. The chimpanzee facility was north-east of the elephant facility. The prevailing wind directions were north-easterly to easterly in summer and north-westerly to westerly in winter.
DISCUSSION
This study describes the transmission of an undistinguishable strain of M. tuberculosis between an elephant and chimpanzee in an Australian zoo, and TST conversion in four people who spent at least 10 h within the elephant enclosure during the elephant's infectious period. One person with a TST conversion also had contact with the chimpanzees during the chimpanzee's infectious period. There were no TST conversions in those who had contact with neither the elephant nor chimpanzee, and no TST conversions in those who had contact only with the chimpanzee. There was no evidence of risk to staff who did not enter the elephant enclosure, or to visitors to the zoo.
M. tuberculosis infections have previously been reported in a wide range of animal species including: elephants [Reference Montali, Mikota and Cheng6, Reference Oh7, Reference Angkawanish22–Reference Mikota, Fowler and Miller24], non-human primates [Reference Isaza, Fowler and Miller5, Reference Montali, Mikota and Cheng6, Reference Miller, Fowler and Miller25, Reference Michel26], rhinoceros [Reference Oh7, Reference Lewerin23, Reference Miller, Fowler and Miller25], tapir [Reference Miller, Fowler and Miller25, Reference Michel26], non-domestic ungulates [Reference Oh7, Reference Lewerin23, Reference Miller, Fowler and Miller25–Reference Lomme27], non-domestic carnivores [Reference Montali, Mikota and Cheng6, Reference Miller, Fowler and Miller25, Reference Alexander28], dogs [Reference Posthaus8, Reference Erwin29–Reference Snider32], cats [Reference Posthaus8, Reference Snider32], guinea pigs [Reference Sakamoto33], rabbits [Reference Sakamoto33], cattle [Reference Fetene, Kebede and Alem34, Reference Ocepek35] and birds [Reference Isaza, Fowler and Miller5, Reference Montali, Mikota and Cheng6, Reference Schmidt36–Reference Washko38]. Susceptibility to infection with M. tuberculosis varies significantly between animal taxonomic groups, with species such as old world monkeys, lesser apes, elephants, hamsters and guinea pigs being highly susceptible; great apes, new world monkeys, canids, tapir, rhinoceros, pigs, rats and mice moderately susceptible; and prosimians, felids, equids, ungulates, rabbits, birds and reptiles having low susceptibility [Reference Isaza, Fowler and Miller5]. The susceptibility for many species remains unknown.
The findings of our study are consistent with other reports [Reference Montali, Mikota and Cheng6, Reference Oh7, Reference Murphree12, Reference Une and Mori13]. M. tuberculosis was diagnosed in two Asian elephants, three Rocky Mountain goats (Oreamnos americanus) and one black rhinoceros (Diceros bicornis) in the Los Angeles Zoo. In that outbreak, TST conversions in humans were associated with working with the elephants and attending an elephant necropsy [Reference Oh7]. In Illinois, after the death of three elephants at an exotic animal farm due to TB and the diagnosis in a fourth live elephant, 22 caregivers at the farm were screened for TB; 11 had positive TST reactions, one of whom was diagnosed with TB disease. Genotyping demonstrated that the isolates from the four elephants and the caregiver with active TB were the same [Reference Maslow10].
Our study was limited by the small sample size and few TST conversions. It was difficult to fully assess the impact of confounding variables and demonstrate a clear association between exposures and outcome. Staff with TST conversions spent at least 10 h inside the elephant enclosure, but some also handled the browse and/or cleaned and maintained the elephant exhibit. Furthermore, tests for TB infection in live animals are difficult to validate and therefore there remains some uncertainty about the exclusion of TB infection using in vitro or in vivo tests.
In humans, TB infection is usually transmitted through aerosols, whereby droplet nuclei containing 1–3 M. tuberculosis bacilli are inhaled and penetrate the lung tissue [Reference Heymann39]. Many of the risk factors identified for contracting TB from a human with active disease are applicable to acquiring infection from an animal, including: number of bacilli being actively shed; total duration of exposure; droplet size; persistence of aerosols through insufficient ventilation; and immune status [Reference Maslow10]. Direct aerosol transmission from the elephant to the staff with TST conversions in our study, seems plausible and likely.
Our inability to fully assess the mode of transmission for the staff with TST conversions, impacted on our ability to determine the pathway of transmission from the elephant to the chimpanzee. Aerosol transmission was considered, particularly in view of the force with which an elephant can expel air from its lungs via the trunk and possibly expel droplets of moisture over long distances [Reference Mikota and Maslow40]. The distance and the presence of buildings and trees between the elephant and chimpanzee enclosures, the prevailing wind directions, and the fact that the chimpanzee were housed in a high-walled enclosure, meant that this mode of transmission seemed improbable as the cause of transmission between the animals.
Indirect aerosol transmission has been documented elsewhere. Five employees working at an elephant refuge in Tennessee had TST conversions linked to the presence of an infected elephant at the refuge. Risk of conversion was increased for elephant caregivers and personnel working in offices connected to the barn. Indirect exposure to aerosolized organisms, poor ventilation and delayed and inadequate infection control practices are likely to have contributed to transmission [Reference Murphree12].
We explored other possible routes of transmission between the elephant and the chimpanzee. There was no evidence of involvement of a human or animal intermediate. Infection was not found in any other animal located between the elephant and the chimpanzee, or any other animals within a 20 m radius of the elephants, nor any staff member working in the air-flow area between the two exhibits. There were no reported cases of TB in humans in NSW or other Australian jurisdictions, with a MIRU profile that matched the strain in the elephant and chimpanzee. Vector transmission of M. tuberculosis has not been documented and no method was available to test this as a likely mechanism of transmission. Browse was occasionally transferred from the pedestrian area behind the elephant barn to the chimpanzees, and staff from other areas of the zoo shared this area. Fomite transmission from the browse would seem a possible route of transmission between the two animals. We were unable to demonstrate that exposure to the browse was an independent risk factor for M. tuberculosis infection, since the three cases who sorted browse all spent at least 10 h inside the elephant enclosure and two of the three had high-level contact with the elephant during its infectious period.
Interpretation of single positive TSTs was difficult due to the potential cross-reactivity of TSTs with mycobacteria other than TB, BCG vaccination and the inability to distinguish recent from remote past infection. Although no association was found between single positive TSTs and elephant and/or chimpanzee exposure, the increase in the relative risk with exposure to the elephant when those people with other risk factors for a positive TST were excluded from the cohort was suggestive of a possible association. The value of baseline TST screening in staff working in areas of potential risk is highlighted.
Our investigation demonstrated the value of systematic risk assessment in the management of TB in animals in our care and their human contacts. Although TB is emerging as a significant disease of elephants in Thailand and other Asian countries, little is known about the transmission dynamics and there is no direct evidence of elephant-generated aerosols. An understanding of potential modes of acquisition of TB from elephants would allow for more targeted and effective measures to prevent transmission from elephants to humans and other animals. Further consideration should also be given to other potential modes of transmission from elephants such as fomites.
ACKNOWLEDGEMENTS
The findings of this study would not have been possible without the efforts of the investigation team, including: authors, Kate Charlesworth, Simon Duffy, Mandy Everett, Jo Griffin, Praveena Gunaratnam, Frances Hulst, Amanda Ivaneza, Peter Jelfs, Lisa Keen, Cameron Kerr, Mary Migan, Jennie Musto, Erin Passmore, Cheryl Sangster, Sue Tattersall, Sean Tobin, Euan Tovey, Kimberley Vinette Herrin, Lee Whiley, Public Health Officer and Biostatistics trainees and supervisors NSW MoH. The authors are grateful to Peter Jelfs and the NSW Mycobacterium Reference Laboratory, ICPMR, Westmead Hospital and scientists from the Department of Microbiology, North Shore Hospital. In addition, we thank Jennie Musto for her input into the study design; and Erin Passmore for her assistance with the literature review. We extend our gratitude to the staff of the zoo for their participation in the study. This research received no specific grant from any funding agency, commercial or not-for-profit sector.
DECLARATION OF INTEREST
None.




