Background
Multidrug-resistant tuberculosis (MDR-TB), defined as a disease caused by infection with Mycobacterium tuberculosis (MTB) strains with resistance to at least isoniazid and rifampin, is a major threat to global TB control efforts [1,Reference Xu2]. In 2017, it was estimated that of 460 000 incident MDR-TB cases, only 25% were actually diagnosed and treated [1]. Because treatment regimens for MDR-TB tend to have low effectiveness, are poorly tolerated and extremely expensive, patients with MDR-TB are more likely to experience poor outcomes and death [Reference Migliori3,Reference Marks4]. In fact, a previous meta-analysis demonstrated that only half of MDR-TB patients treated with second-line regimens achieved successful clinical outcomes after treatment completion [Reference Orenstein5]. Treatment failures of MDR-TB cases not only resulted in an increased hazard ratio for death, but also accelerated the transmission of this form of TB within the community [Reference Gandhi6].
China has been described as a global ‘hotspot’ of MDR-TB, accounting for 11% of disease burden worldwide [1,Reference Zhao7]. According to a national survey on drug-resistant TB, MDR-TB represents 5.7% of new cases and 25.6% of previously treated cases in China [Reference Zhao7]. The high rate of MDR-TB in this country continues to reverse recent strides made in recent decades toward reducing TB morbidity and mortality. More importantly, despite the serious threat posed by MDR-TB, insufficient attention has been paid to diagnose and treat MDR-TB patients [Reference Zhao7]. In China, a policy of free drug treatment for TB covers the costs of an entire course of first-line anti-TB drugs, while the extremely expensive second-line anti-TB drugs for MDR-TB treatment must be paid for by health insurance and patient payments of out-of-pocket costs [8,Reference Long9, ]. Therefore, a sizeable proportion of MDR-TB patients incur catastrophic financial hardship stemming from the high costs of TB medical care that they frequently cannot afford, resulting in unplanned treatment interruption [Reference Wei10]. This situation has undoubtedly contributed to the increasing mortality and transmission dynamics of MDR-TB epidemics in China. Consequently, several studies have focused on factors contributing to the high case fatality rate of the MDR-TB patient cohort [Reference Xu2,Reference Tang11], but data on patient survival and factors influencing the length of survival are limited, especially in settings with high prevalence rates of MDR-TB.
To address this issue, here a retrospective study was carried out among all MDR-TB patients in Wuhan, the largest city in Central China. We analysed the survival of a cohort of MDR-TB patients over a 10-year period (2006–2016). Our aim was to evaluate long-term survival and risk factors associated with the reduced survival of MDR-TB patients.
Methods
Setting and study population
Wuhan, the capital of Hubei Province, has an area of 8494 km2 and a population of 10.91 million. In 2010, Wuhan had a TB case notification rate of 436.3 per 100 000 people per year, with 6.9% of new cases and 18.8% of previously treated cases diagnosed with MDR-TB [Reference Qin12]. This retrospective 10-year study was conducted in Wuhan Pulmonary Hospital, the hospital supervising TB control activities in Wuhan. Both new (incident) and retreatment MDR-TB adult patients were enrolled in the present study after diagnosis using conventional in vitro drug susceptibility testing (DST). All patients received follow-up care for 5–10 years after diagnosis. Demographic and clinical data were extracted from the National Tuberculosis Registry database and from patient medical records held at Wuhan Pulmonary Hospital. Follow-up data on the survival and cause of death were obtained by interviewing patients or their relatives via telephone once a year. Briefly, the patients were first interviewed via telephone, and those who could not be found by telephone calls were interviewed with their relatives via telephone. The censored categories refer to: (i) loss to follow-up; (ii) survival by the end of follow-up period; (iii) death from a competing risk.
Laboratory examination
Sputum specimens were submitted to the laboratory for mycobacterial culture as previously described [Reference Pang13]. Bacterial colonies were harvested 4–8 weeks and subjected to conventional in vitro DST and MTB species identification testing [Reference Pang13]. The drugs tested in these studies included isoniazid, rifampin, ethambutol, streptomycin, kanamycin and ofloxacin. Pre-extensively drug-resistant tuberculosis (pre-XDR-TB) was defined as a disease caused by MDR-TB isolates with additional resistance to either one fluoroquinolone (FQ) or one second-line injectable drug (SLID) but not both. XDR-TB was caused by MDR-TB isolates that were additionally resistant to one FQ and one SLID.
Treatment
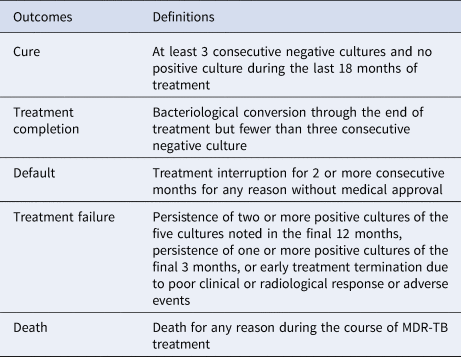
From December 2006 to June 2011, MDR-TB patients received a standardised antibiotic treatment regimen previously developed by the Global Fund. The abbreviation of each drug was as follows: Am, amikacin; Cm, capreomycin; Cs, cycloserine; E, ethambutol; Lfx, levofloxacin; Mfx, moxifloxacin; PAS, p-aminosalicylic acid; Pto, protionamide; Z, pyrazinamide. Treatment consisted of a 6-month intensive phase followed by an 18-month continuation phase of: 6 Z, Am (Cm), Lfx (Mfx), Cs (PAS, E), Pto/18 Z, Lfx (Mfx), Cs (PAS, E), Pto. Treatment outcomes were categorised according to the standard defined WHO terms that included cure, complete, death during treatment, failure, loss to follow-up and not evaluated [Reference Laserson14,Reference Wang15](Table 1).
Table 1. Treatment outcome definitions in MDR-TB patients
Statistical analysis
Double entry of data was conducted using the EpiData Software Package (EpiData Assoc., Odense, Denmark). Death was defined as mortality from TB during anti-TB treatment or follow-up periods. The χ 2 analysis of variance tests were used to compare categorical variables. Case fatality rates (per 100 person-years (PY)) and Kaplan–Meier survival curves were analysed to estimate survival rates. We used crude and adjusted Cox proportional hazard regression analysis to identify and evaluate the factors impacting survival, including demographic characteristics, initial drug susceptibility profiles and clinical treatment. Death from MDR-TB was designated as follow-up termination. In addition, variables were selected using a forward LR method and remained in the model for a P value of <0.1. The adjusted hazard ratio (aHR) was calculated to describe the relative hazards affecting MDR-TB patient survival. SPSS software version 20.0 (SPSS Inc., Chicago, IL) was used for all analyses. A P value of <0.05 was considered statistically significant.
Results
Demographics
A total of 356 MDR-TB cases reported in Wuhan during 2006–2011 were included in this study. Cases included 262 male and 94 female patients that together had an overall mean age (range) of 46.45 (16–78) years. Of these study subjects, 165 (46.3%) were unemployed. A previous history of TB disease was reported by 302 patients. Previously treated MDR-TB cases had undergone a mean of 1.83 failed courses of treatment. The most frequent comorbidity in this study was diabetes (46/103, 44.7%), followed by cirrhosis (6/103, 5.8%) and kidney disease (2/103, 1.9%). HIV tests were conducted on all patients and no patients were found to be seropositive. Patients were infected with organisms that were resistant to a mean of 3.48 (2–6) drugs, with 104 and 12 patients diagnosed with pre-XDR-TB and XDR-TB, respectively (Table 2).
Table 2. Mortality of multidrug-resistant tuberculosis cases stratified to demographic and clinical characteristics

a MDR, multidrug-resistance, no additional resistance to FQs or second-line injectable drugs; XDR, extensive drug-resistance; pre-XDR, MDR plus additional resistance to either any FQ or any second-line injectable drug but not both.
Of 356 MDR-TB cases, 231 (64.9%) received treatment with second-line drug treatment regimens, while the remaining 125 (35.1%) refused treatment in the Wuhan Pulmonary Hospital. Among 231 cases undergoing treatment, 167 (72.3%) were cured, two (0.9%) died, 31 (13.4%) defaulted and 31 (13.4%) failed treatment. Of the 31 patients who had defaulted treatment, the average treatment period was 7.8 months (95% CI 5.5–10.2 months).
Survival analysis
The total follow-up period was 270 PY,the mean follow-up time was 2.3 (1.25–3.67) PY. Of the entire patient sample, 103 patients died, yielding an average case fatality rate of 381.2 per 1000 TB patients per year (95% CI 314.25–462.4). Case fatality rates were highest among patients with diabetes, those in the rural population and patients infected with pre-XDR/XDR, whereas the lowest case fatality rates were observed among unmarried patients and new TB cases.
Using adjusted Cox regression analysis, older age (aHR >3.0 starting from 30 years) and low education level (primary and middle school; aHR 1.67 (95% CI 1.01–2.77)) were independent factors associated with lower survival. Previous treatment history profoundly affected the survival of MDR-TB patients (aHR 13.15 (95% CI 1.82–94.96)) (Fig. 1). In addition, patients with diabetes mellitus were associated with a higher risk of dying during the follow-up period (aHR 1.95 (95% CI 1.30–2.93)). As a factor independent of the aforementioned factors, the patients refusing treatment were at elevated risk for mortality in our observation (aHR 2.98 (95% CI 1.95–4.54)). In contrast, adjusted analyses revealed that gender, residence, marriage status or occupation had no significant effect on survival (Table 3).

Fig. 1. Kaplan–Meier overall survival curve of 356 MDR-TB patients according to the drug-resistant pattern.
Table 3. Factors associated with mortality among the total MDR-TB cases
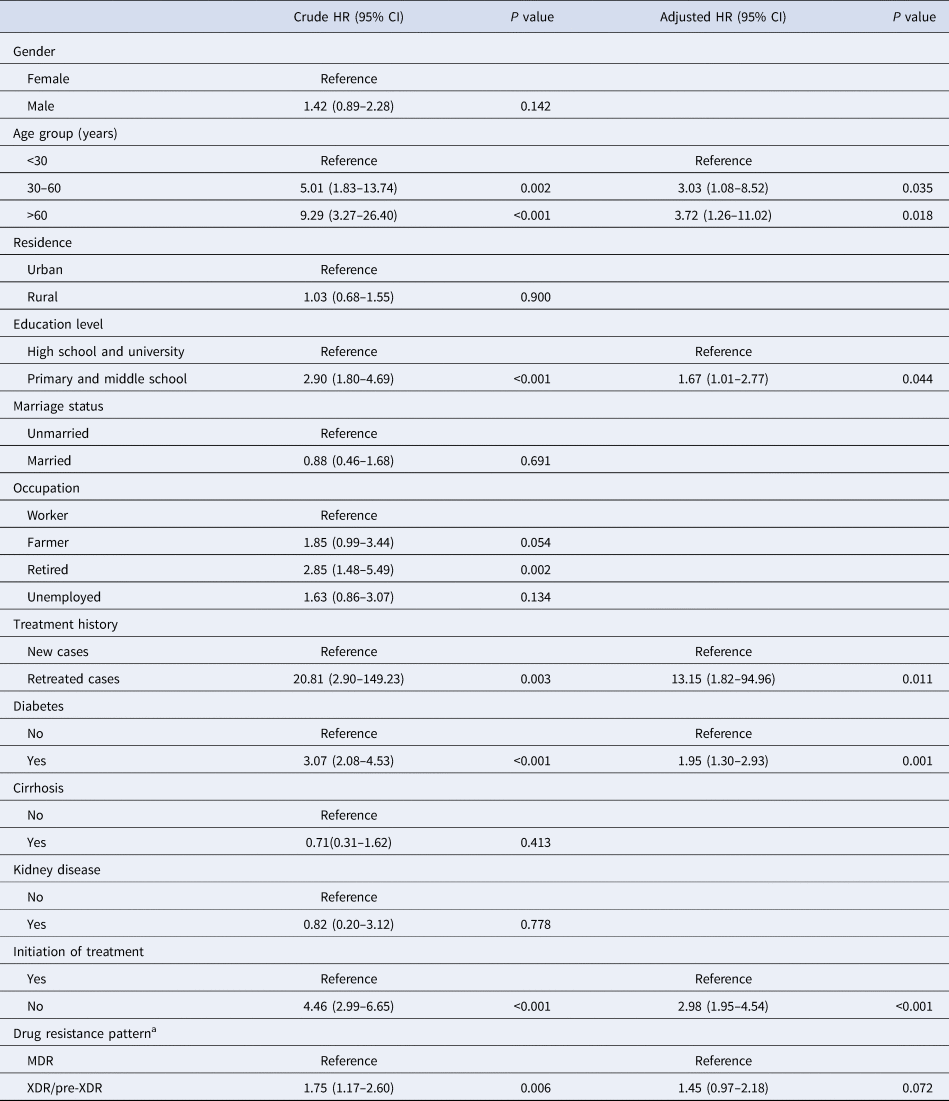
HR, hazard ratio; CI, confidence interval.
a MDR, multidrug-resistance, no additional resistance to FQs or second-line injectable drugs; XDR, extensive drug-resistance; pre-XDR, MDR plus additional resistance to either any FQ or any second-line injectable drug but not both.
Discussion
Although TB mortality has undergone a progressive decline worldwide over the past few decades, TB remains to be one of the leading causes of death from communicable disease, while also markedly contributing to the epidemic of MDR-TB [1]. A decline in mortality from MDR-TB would not only depend on decreased death rate during anti-TB therapy, but also on the achievement of long-term survival after treatment. In this study, we first identified the factors associated with the long-term survival of MDR-TB patients in a resource-limited medical care setting. Our data demonstrate that coexisting diabetes had a significant negative impact on the survival of MDR-TB patients. However, these results do not align with the results of a recent study by Siefert and coworkers using follow-up data of patients with suspected drug-resistant TB; that study failed to show any association between survival and diabetes status [Reference Seifert16]. This discrepancy in results may be due to a small sample size stemming from the low diabetes prevalence among patients in that study [Reference Seifert16]. Meanwhile, a series of other reports also have demonstrated that diabetes confers an increased risk of treatment failure and death compared with the corresponding risk for non-diabetic patients [Reference Baker17]. Moreover, TB patients with diabetes also appear to have a high risk of relapse, even after achieving favourable outcomes [Reference Ma18,Reference Dooley19]. Thus, when taken together, high rates of treatment failure and post-treatment relapse appear to result in an overall high case fatality rate for diabetic TB patients. We speculate that diabetic status, associated with hyperglycaemia and poor glucose control, leads to a dysfunctional immune response to MTB in diabetic patients, which may explain much of the association between diabetes and increased risk of death [Reference Dooley19]. Consequently, our results highlight that optimised glucose control should be an essential component of diabetic patient treatment management to maximise the chances of prolonged survival (Table 1).
It is notable that MDR-TB patients aged 60 years or older had a greater risk of mortality during follow-up. In fact, several experts have suggested that high TB mortality in the elderly may be associated with weakened immunity and a greater number of comorbidities [Reference Hochberg20,Reference Negin21]. In our cohort, this association continued to be observed even after we adjusted for additional comorbidities. This finding suggests that older MDR-TB patients experienced a higher likelihood of death due to waning immunity associated with advanced age. Another potential explanation for the high case fatality rate in the elderly is that they may, as a group, experience particularly delayed diagnosis and treatment. Moreover, numerous researchers have revealed that elderly TB patients tend to exhibit non-specific disease presentation at TB onset, such as negative bacterial detection results and ambiguous clinical findings, which could delay diagnosis [Reference Yoshikawa22,Reference Dahmash23]. Although AFB smear testing is the standard laboratory method used for TB screening, more effective diagnostic methods with improved sensitivity are needed for the testing of paucibacillary sputum samples collected from elderly patients to achieve earlier detection of MTB. Earlier detection would likely minimise interventional delays to improve elderly patient treatment outcomes.
Another important finding of this study was that more than one-third of MDR-TB patients refused anti-TB treatment. Although the exact reasons underlying patient refusal of treatment remain unclear, lack of treatment would undermine control measures to reduce TB prevalence within the community, while also preventing mortality in these cases. Furthermore, untreated MDR-TB patients would remain a reservoir for TB transmission by supporting bacterial propagation that would likely accelerate the transmission of MDR-TB to the community. Therefore, to ultimately control TB in the community, more effective strategies are needed to address this public health concern at the level of the individual patient in China.
Our study had several obvious limitations. First, because in vitro second-line DST results for only KAN and OFLX were obtained in this study, incomplete DST results made it impossible to assess the impact of cross-resistance to FQs and SLIDs on treatment outcomes. Second, despite observations that additional drug resistance is an independent risk factor associated with the mortality of MDR-TB patients [Reference Falzon24], low prevalence of pre-XDR- and XDR-TB in our cohort reduced our ability to evaluate the correlation between different drug resistance profiles and long-term survival. Third, since this study was conducted at only a single site, our findings should therefore be verified using larger epidemiological and clinical studies of patients from geographically diverse sites. Fourth, although this study demonstrated an association between diabetes comorbidity and decreased survival, diabetic severity was not investigated for its impact on long-term survival. Finally, it is an interesting topic to assess a difference in case fatality rates during and after treatment, whereas only three patients died during the treatment period. Hence, the small sample size was insufficiently large for statistical analysis. Despite these limitations, this study provides new insights to increase the understanding of risk factors contributing to the mortality of patients living in an area of high TB prevalence. Such studies are essential for formulating appropriate strategies to improve high-risk patient outcomes.
Conclusion
In this study, we demonstrated that coexistent diabetes had a significant negative impact on the survival of MDR-TB patients. Moreover, the presence of XDR- and pre-XDR-TB disease, irrespective of FQ- or SLID-resistance, led to poorer long-term survival compared with the survival of MDR-TB patients. In addition, MDR-TB patients aged 60 years or older had a greater risk of mortality during follow-up. Our findings suggest that MDR-TB patients with certain comorbidities should receive treatment interventions aimed at reducing their particularly high risk of mortality.
Acknowledgement
We are very grateful to the support and assistance of the TB prevention and control institutions at the urban level in Wuhan.
Ethical standards
This study was approved by the Ethics Committee at Wuhan Pulmonary Hospital. Written informed consent was obtained from individuals before an interview was conducted.






