Introduction
Dengue is the most important human arbovirus disease worldwide [Reference Martinez1]. Four serotypes have been described, DENV-1, DENV-2, DENV-3 and DENV-4, which can manifest a variable clinical spectrum, ranging from asymptomatic to severe forms [Reference Sabchareon2]. Approximately 2–5 billion people live in 100 countries where dengue is endemic, situated in tropical and subtropical areas [3]. In 2013, the world estimate for dengue virus infections was 390 million persons, with 96 million symptomatic cases [Reference Bhatt4].
The epidemiological profile of dengue varies around the world. In Southeast Asia, it mainly affects children, as opposed to adults in the Americas [Reference Halstead5]. Brazil has witnessed three dengue epidemics in the last 12 years, in 2002, 2008 and 2010, with a predominance of serotypes DENV-2 and DENV-3. Until 2006, dengue affected almost exclusively adults and participated very little in the differential diagnoses of common acute febrile diseases in childhood. In the 2007–2008 epidemic in the city of Rio de Janeiro, the reintroduction of DENV-2 virus resulted in greater severity and a large increase in the number of cases in children, posing a clinical challenge for more adequate diagnostic and therapeutic criteria in pediatrics, especially in infants [Reference Barreto and Teixeira6].
There are several hypotheses for more severe dengue in individuals or populations. One is the sequential infection theory, according to which in the presence of antibodies against a specific serotype, new infection with another serotype triggers an exacerbated inflammatory response [Reference Halstead5]. Other hypotheses include greater virulence of certain strains, particularly DENV-2 [Reference Chen7, Reference Halsey8], individual factors like chronic diseases [Reference Nguyet9] or genetic factors [Reference Blanton10, Reference Monteiro11] and age bracket. Evidence suggests that infants may evolve more rapidly to shock [Reference Trung12], while adults have a greater tendency to bleeding and severe organ impairment. A study in Nicaragua compared the clinical presentation by age bracket and found higher incidence in children 5–9 years of age and greater severity in infants [Reference Hammond13].
In Brazil, dengue is a disease of compulsory notification and all suspected and confirmed cases must be reported to the Municipal Health Secretariats, the government agency responsible for epidemiological surveillance at the local level [14, 15].
Most studies on dengue spatial distribution have emphasised the importance of climate in the occurrence of epidemics. Few studies have compared the incidence, clinical presentation and severity between age brackets, particularly in Brazil [Reference Teixeira16]. This study thus aimed to provide a clinical characterisation of severe dengue cases and spatial distribution of incidence and case-fatality rates according to age bracket.
Methods
Rio de Janeiro is a metropolis with an area of extended influence. The city is located in Southeast Brazil and has an area of 1224.56 km2, characterised by a long coastal strip interspersed with mountains, lagoons and lowlands, an environmental diversity that gives it great tourist potential. The city of Rio de Janeiro had a population of 6 320 446 in 2010.
For purposes of planning activities and services, including in health, the city of Rio de Janeiro was subdivided according to geographic area and population density into ten Program Areas (PA): PA 1.0 – Downtown, PA 2.1 – South Zone, with several long beaches, PA 2.2, PA 3.1, PA 3.2 and PA 3.3 – located in the North Zone, interspersed with mountains and PA 4.0, PA 5.1, PA 5.2 and PA 5.3 – in the West Zone, with extensive areas of vegetation and bordering on the coastline. The areas have distinct socioeconomic and health characteristics (Table 1). The human development index (HDI) summarises the social indicators income, schooling and health [17]. Highest values (0.93) are found in PA 2.1, representing very high human development (HDI >0.8) and lowest values (0.75) in PA 5.2, meaning high human development (0.700<HDI<0.799) (UNDP, 2016).
Table 1. Population of the Municipality of Rio de Janeiro by age bracket and HDI according to Program Areas

HDI, human development index.
Source: Brazilian Institute of Geography and Statistics (IBGE). 2010 Population Census.
This ecological study used the database from the National Information System for Diseases of Notification (SINAN), which consolidates the data collected from the Individual Notification Form (FIN), standardised for the entire country.
The study included all dengue cases residing in the city of Rio de Janeiro and reported in 2007 and 2008. Confirmed cases were defined as meeting the following criteria from the Brazilian Ministry of Health (2005) [18]: positive sample for detection of viral RNA by RT-PCR (reverse transcriptase, polymerase chain reaction), ELISA (capture enzyme-linked immune sorbent assay) for IgM antibodies; IgM or IgG seroconversion in paired samples by ELISA; or the clinical-epidemiological criterion, namely a consistent clinical picture, epidemiological link to a confirmed dengue case and absence of laboratory confirmation of other diagnoses consistent with the age bracket. The study excluded cases that were treated in the city of Rio de Janeiro but resided elsewhere, those with missing data and those with diagnostic confirmation of other acute febrile diseases.
During the study period, Brazil used the WHO classification from 1997 [19]: dengue fever (DF), dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS), plus the category ‘dengue with complications’ for cases that met some but not all the criteria for DHF [18, 20].
In the final sample, we analysed the following variables: socio-demographic – age, sex, colour (white/non-white) and neighbourhood of residence; clinical–final case classification (dengue fever, dengue with complications and DHF) and final outcome (discharge/death); and laboratory – median platelet count (thousand/mm3). For cases classified as ‘dengue with complications’ or ‘dengue haemorrhagic fever’, we analysed the presence of haemorrhagic manifestations, plasma leakage (evidenced by rising haematocrit, cavitary effusion, or hypoproteinemia) and other complications (neurological alteration, respiratory dysfunction, liver failure, platelets <50 000/mm3, digestive tract bleeding, cavitary effusions and white blood cells <1000/mm3) and hospitalisation (yes/no). Laboratory variables included the isolated viral serotype and platelet count.
The analyses were stratified by age bracket: infants (<2 years), preschoolers (2–5 years), schoolchildren (6–11 years), adolescents (12–17 years) and adults (⩾18 years). Differences in proportions were evaluated using the Pearson χ 2 test (or Fisher's Exact test) with significance set at P < 0.05. When the proportion of missing data exceeded 20%, the distribution by age, sex and hospitalisation of losses was compared with the final sample using the Pearson χ 2 test (P < 0.05). The analyses used SPSS, version 16 [21].
Dengue incidence and case-fatality rates were calculated using the 2010 population census base from the Brazilian Institute of Geography and Statistics (IBGE) [22]. Spatial distribution of dengue incidence and case-fatality rates by age bracket in the city of Rio de Janeiro (according to program area) was explored with thematic maps. We used the neighbourhood connectivity structures, k = 2, k = 4 and Delaunay triangulation geometric matrices, the sphere of influence, relative neighbourhood graph and Gabriel graph. Spatial autocorrelation was explored using the global and local Moran and Geary indices. Positive autocorrelation means similarity between areas, while negative values denote distinct areas. Significance was set at 0.05. Influential points and outliers were evaluated by the Moran plot. Local Moran maps were based on LISA (Local Indicators of Spatial Association). The spatial analysis used the spdep, splancs and maptools libraries of the R free software, version 3.2.3 [23].
The study was approved by the research ethics committees of the Evandro Chagas National Institute for Infectious Diseases (INI) and the Rio de Janeiro Municipal Health Secretariat. The study used routine secondary data collected by the Epidemiological Surveillance System, analysed anonymously and consolidated to ensure patient secrecy.
Results
From 2007 to 2008, 159,887 suspected dengue cases were reported in the city of Rio de Janeiro, of which 151 527 resided in the city. A total of 5281 cases were excluded in which the diagnosis of dengue was ruled out and 107 438 (70.9%) with unknown clinical classification. The final sample included 38 808 cases (Fig. 1).

Fig. 1. Flowchart of the Study.
The final sample had proportionally more children 6–11 years of age when compared with the losses (19.8% and 15.1%, respectively; P < 0.001). There was no difference according to sex (P = 0.348) or hospitalisation (P = 0.499).
The majority of the cases were females (54.0%) and white (51.1%); 42.4% of cases were individuals under 18 years. Of all the cases classified as haemorrhagic dengue, 61% were younger than 18 years, the highest proportion of which in schoolchildren. The confirmatory criterion was predominantly clinical-epidemiological (60.7%). Clinical evolution was missing in 10 551 cases (27.2%) (Table 2).
Table 2. Description of sample according to demographic and clinical variables, City of Rio de Janeiro, Brazil, 2007–2008
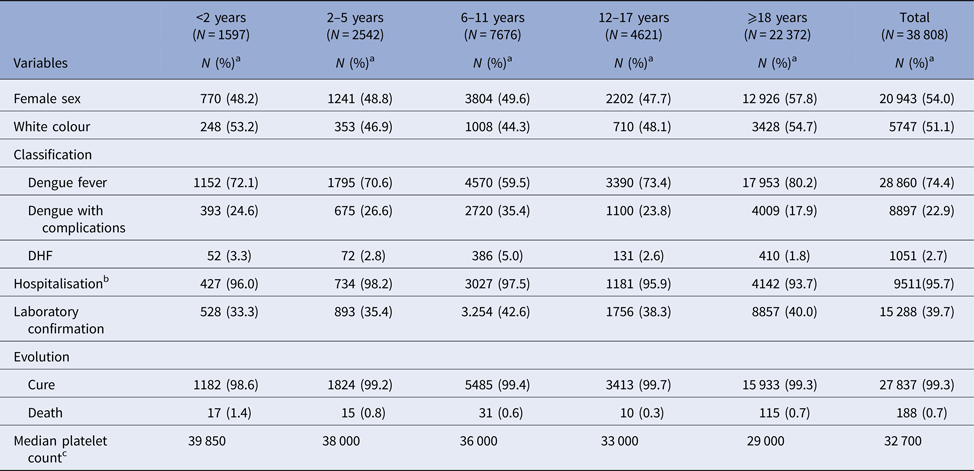
DHF: dengue haemorrhagic fever.
a % in column.
b Percentage in column for total number of severe cases (dengue with complications plus DHF).
c Thousand/mm3.
Among the cases confirmed by the laboratory criterion (n = 15 288), viral isolation was performed in 135 cases, of which 94 were DENV-2 and 41 DENV-3. Of all deaths from dengue (188 cases), 187 occurred with severe forms of the disease; 106 cases in dengue with complications and 81 cases from DHF. The case-fatality rate was 1.3% in DF and 7.7% in DHF.
Hospitalisation occurred in 95.7% of the severe forms (dengue with complications and DHF). Clinical variables and median platelet count among severe cases were analysed according to age bracket (Table 3). Haemorrhage occurred in all the age brackets, with petechiae as the principal sign/symptom. Plasma leakage was more frequent than haemorrhage, in the form of rising haematocrit in adults and adolescents and cavitary effusions in infants and preschoolers.
Table 3. Clinical characteristics of dengue cases with complications or dengue haemorrhagic fever according to age bracket

*χ 2 test **Fisher's Exact Test ***per mm3 ****11 cases of liver failure, 3 with leucocytes <1000/mm3 and 2541 with signs unrelated to DHF.
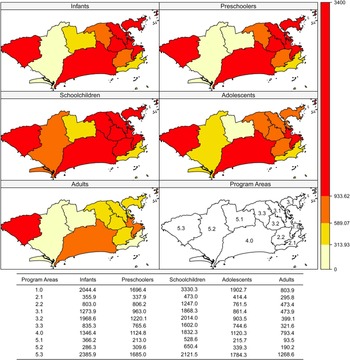
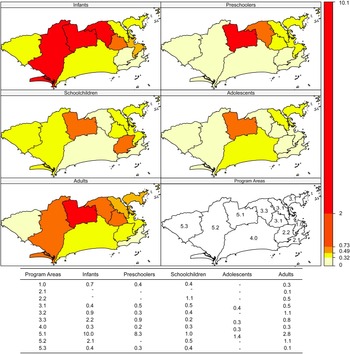
Incidence rates were higher in areas 5.3, 1.0 and 4.0 and in schoolchildren and infants (Fig. 2). Case-fatality was higher in area 5.1 in all age brackets except schoolchildren (Fig. 3).
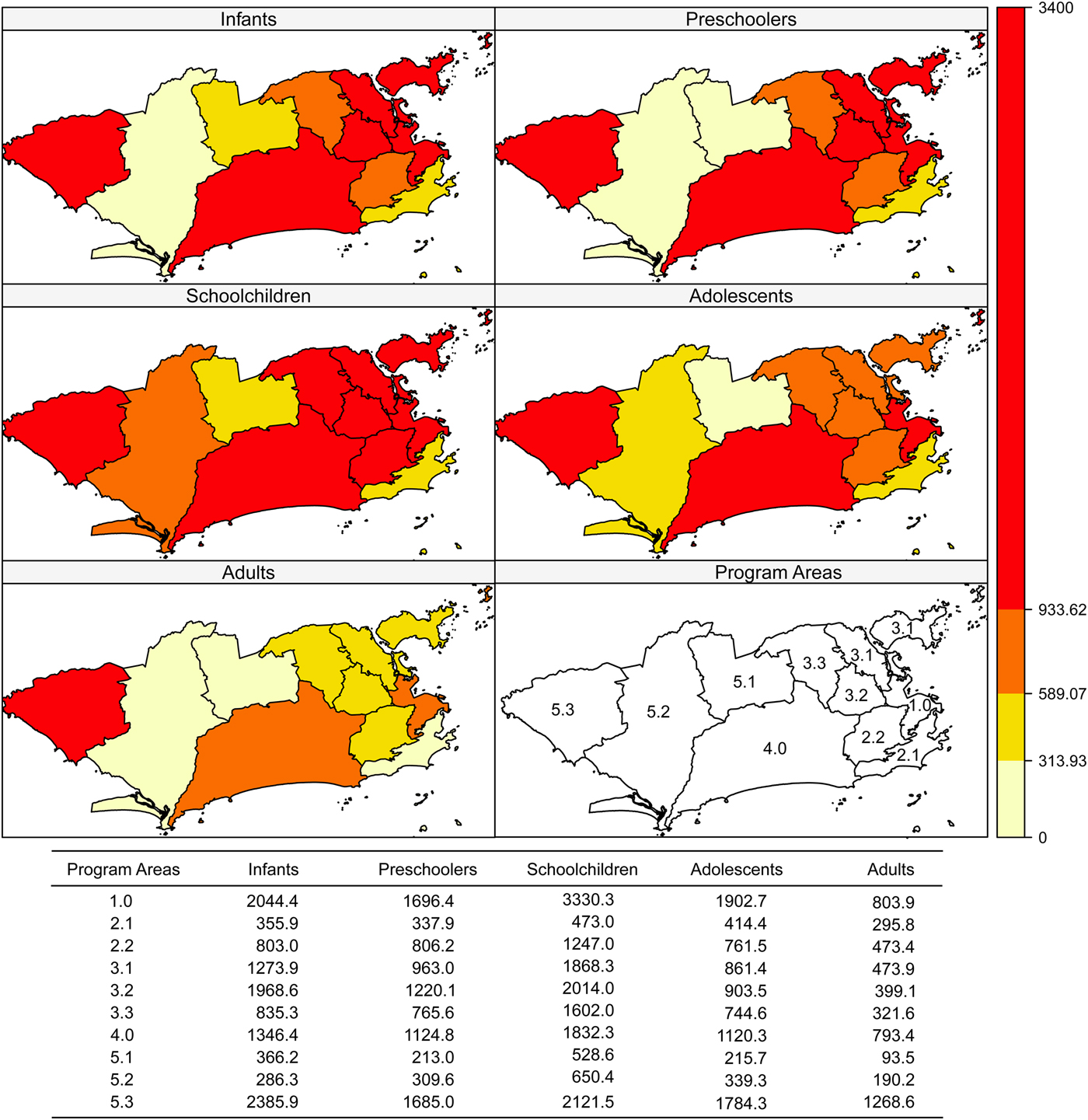
Fig. 2. Dengue incidence rates (per 100 000 inhabitants) according to age bracket and Program Area, Rio de Janeiro, Brazil, 2007–2008.
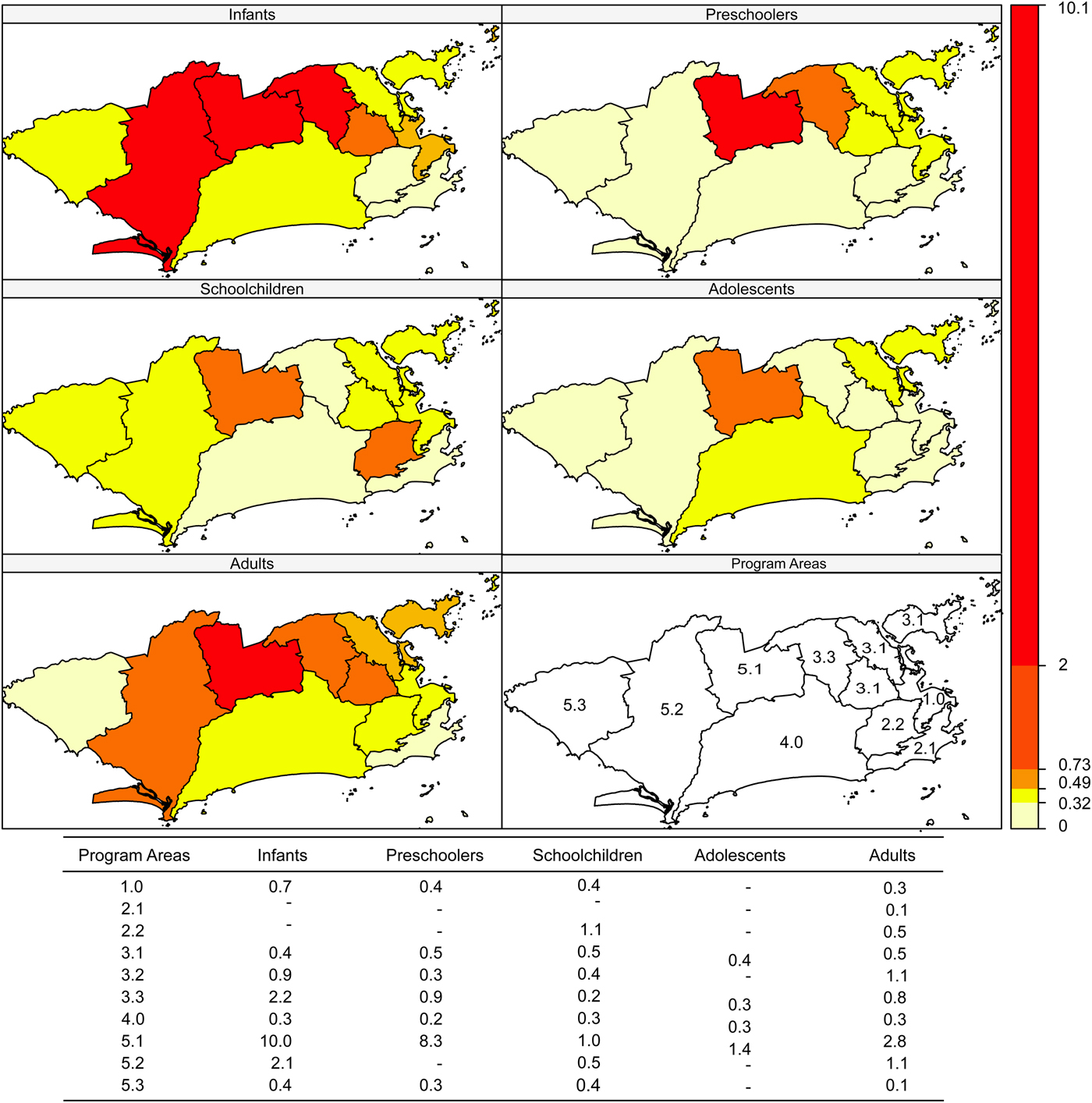
Fig. 3. Dengue case-fatality (%) according to age bracket and Program Area, Rio de Janeiro, Brazil, 2007–2008.
Dengue incidence did not show significant global spatial autocorrelation in any age bracket for any type of neighbourhood matrix. Case-fatality showed significant spatial autocorrelation according to the global Moran and Geary indices.
As for the local Moran (including the graph) and Geary indices, negative spatial autocorrelation was observed for incidence in PA 5.3 in all age brackets. Therefore, PA 5.3 has a different incidence rate from its neighbours: PA 5.2 (all matrices), PA 5.1 (k = 2, k = 4 and triangulation), PA 3.3 and PA 4.0 (k = 4 and Delaunay triangulation). In infants, the incidence in PA 5.3 was higher than in PA 5.1, PA 5.2 and PA 4.0, which was observed with all the neighbourhood matrices (P-values from 0.004 to 0.043). For preschoolers and adolescents, this finding was limited to the k = 2 (P = 0.003), k = 4 (P = 0.034) and triangulation (P = 0.034) matrices. Schoolchildren presented higher incidence in PA 4.0 compared with 5.1 and 3.3, only detected by the k = 2 matrix (P = 0.005).
Case-fatality showed negative spatial autocorrelation in PA 4.0 in infants (relative matrix, P = 0.022), in PA 5.1 in preschoolers (P-values from 0.012 to 0.022, except for the k = 2 matrix), in PA 2.2 (all matrices, P-values from 0.001 to 0.012) and in PA 2.1 (k = 2, sphere, relative and Gabriel, P-values 0.042, 0.042, 0.001 and 0.042, respectively) in schoolchildren and in PA 5.3 in adults (k = 2, P = 0.018).
Discussion
This was a retrospective study that used data from the Information System for Diseases of Notification (SINAN) collected during a dengue epidemic. The fact that losses showed a higher proportion in the 6–11-year age bracket may have introduced a bias, overestimating the data for this age bracket. However, our results were similar to those of other studies [Reference Hammond13, Reference Siqueira24]. Laboratory confirmation was possible in 39.4% of all cases and in 100% of cases of DHF, since it is one of the required criteria for this classification. This probably occurred because of the epidemic period, with a high volume of suspected cases. To avoid this selection bias, we chose to include the clinical-epidemiological confirmatory criterion adopted by the Brazilian Ministry of Health. The study period was during an epidemic, which may have negatively influenced the data quality due to the overload on the system. A reliability study on the final diagnosis of dengue for cases reported to SINAN during an epidemic (2001–2002) showed a high proportion of information keyed in with the specific code ignored or simply with the field left blank [Reference Toledo25]. Notification forms are completed at all levels of health care. A study that evaluated the quality of dengue epidemiological surveillance data in Belo Horizonte, Minas Gerais, Brazil, found 37% under-recording, even among hospital inpatients, from 1997 to 2002 and the rate was eight times higher in hospitals outsourced by the public health system as compared with government hospitals. The system's sensitivity was 63% and the positive predictive value was 43%. Severe cases showed the highest notification rates [Reference Duarte and Franca26].
There was an increase in the number of cases and in the incidence rates in children up to 11 years of age, with more severe clinical presentations than in adults. In this epidemic, signs of plasma leakage were more frequent than bleeding in patients less than 18 years of age. Rising haematocrit was the most frequent sign among cases of DHF, affecting the majority of adolescents and adults (>80%). Cavitary effusions and DSS were more frequent in children less than 11 years of age and hypoproteinemia was more frequent in infants. Although the magnitude of the disease was greater in the central area of the city and in Program Area 5.3, the latter with significantly discrepant values compared with the neighbouring areas in the West Zone of the city, PA 5.1 in the West Zone called attention due to its high case-fatality in preschoolers when compared with the neighbouring areas.
The increases in the number of dengue cases and severe forms in children under 15 years of age corroborate the reports by Barreto and Teixeira [Reference Barreto and Teixeira6] and agree with other findings in the Americas, although to a lower degree than in Asia [Reference Teixeira16]. Circulation of serotype DENV-3 and of the two different serotypes in the same epidemic (DENV-2 and DENV-3) may have contributed to this greater severity [Reference Halstead5, Reference Chen7, Reference Wichmann27]. The hospitalisation rate was 95.7%% in dengue cases with complications or DHF. However, this high rate could be explained by the fact that it was an epidemic, with an increase in severe forms of the disease and pediatric cases. Other studies have shown similar results [Reference Hammond13, Reference Siqueira24].
Haemorrhagic manifestations were more frequent in adults. Among patients less than 18 years of age, schoolchildren and adolescents showed the most bleeding. This could be explained by the fact that platelet count in adults was lower than in children, corroborated by lower median platelet count in groups with more episodes of bleeding.
Other studies have corroborated more frequent rising haematocrit in adults and cavitary effusions in schoolchildren. A study in Vietnam and Thailand also showed more frequent evolution to DSS in children [Reference Trung12, Reference Kittigul28]. A study in Nicaragua found more bleeding in adults and more plasma leakage in children; however, among the latter, infants were more affected [Reference Hammond13]. However, in a study in Thailand, the age group that evolved most frequently to shock was schoolchildren. In our study, evolution to DSS in infants and preschoolers occurred in a similar proportion to that of schoolchildren [Reference Witayathawornwong29].
The types of clinical complications (among those possible in dengue with complications) differed between age brackets. Neurological alterations, cardiorespiratory dysfunction and platelet count <50 000/mm3 were more frequent in adults. Cavitary effusion and digestive tract bleeding in patients under 18 years, but predominantly in schoolchildren and adolescents and in isolation in adults, contributed to 45.5% of all these cases of bleeding. The least frequent complication was a neurological alteration. The literature includes other studies reporting that neurological complications are uncommon, occurring predominantly in adults [Reference Joob and Wiwanitkit30–Reference Weeratunga32].
Nearly all of the deaths occurred in severe cases, with only one death in dengue fever. The highest case-fatality rate was in DHF, with 7.7%, considered high even for severe cases [3]. When stratified by age bracket, infants had the highest case-fatality rate, with 1.4%. The epidemic period and change in the age profile, with an increase in the number of cases in children, presenting a disease whose clinical management was not common among pediatricians, may have contributed to this high case-fatality rate. Another study found these same factors, in addition to greater vulnerability in infants, as possible causes for this high death rate [Reference Teixeira16, Reference Malhão33].
When the autocorrelation between PAs was analysed, PA 5.3 showed a particularly high incidence rate, which could be explained by the extensive areas with vegetation, including environmental protection areas. Despite the high incidence in Program Area 1.0, a central area with people circulating from all the city's neighbourhoods, including those from areas with high dengue incidence, this same PA 1.0 had the lowest case-fatality rate (<1%) in all age brackets, probably due to the higher concentration of health services, including those with greater complexity of care, plus the fact that it is well-served by the public transportation system, facilitating access to health services. Meanwhile, in PA 5.3, despite having fewer health services, case-fatality was also <1%, suggesting better articulation between the existing services. In the opposite direction, although the incidence was lower in PA 5.1, this area showed the highest case-fatality rate. Differences in local infrastructure conditions and health services staffing (and in management) may have contributed to this unfavorable outcome, since the HDI, number of health units and transportation system are equivalent to those of areas 5.1 and 5.3 [34]. PA 3.2, which is part of the North Zone and has a higher population density, showed high incidence in infants, preschoolers and schoolchildren, but with case-fatality <1% in the same age brackets and 1.1% in adults, the second highest value.
This study's strengths include the large sample size, enabling analysis of routine epidemiological surveillance data for the city and the scope of the database, allowing analysis of differences in the clinical profile in five age brackets, from infants to adults. As far as we know, this is the only study that assessed the clinical variability between adults and children in such detail, as well as the potential formation of clusters for any of the target morbidity and mortality indicators.
The SINAN database (Information System for Diseases of Notification) is an instrument for data consolidation used both in epidemiological surveillance and in the generation of information to orient public dengue control guidelines. Data quality generates adequate information and should be assessed systematically. During epidemics, it is plausible to assume some over-reporting, especially of more severe cases, together with an increase in the proportion of blank or unknown items in reporting forms.
There are clinical and laboratory differences in dengue between adults and children and between various age brackets in children. Adults are more prone to bleeding and low platelet count, while children tend to present vascular leakage. The severe forms of the disease and deaths occurred predominantly in children. The study's sample is homogeneous and includes a large number of cases. The geographic characteristics of the Program Areas could explain the variations in dengue incidence between them. However, the discrepant case-fatality rates in areas with similar populations, health services and accessibility raise a warning flag concerning the possible need for better training, management and monitoring of health professionals involved in dengue patient treatment in areas with higher case-fatality. In the absence of efficacious vaccines to date, the control of dengue morbidity and mortality depends on well-targeted public policies. More studies are needed, especially prospective studies evaluating different presentations between adults and children and comparing pediatric age brackets. Systematic evaluation of the instruments that consolidate data on dengue is important for generating adequate information and thus an accurate grasp of the problem and appropriate resolution.
Acknowledgements
We thank the staff of the Health Department of the Rio de Janeiro city who work to improve the quality of the free available databases. This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (Grant number 110.188/2014 and E-26/110.964/2013). YHMH was supported by Estácio de Sá University (Pesquisa Produtividade 1352017).
Declaration of Interest
None.