INTRODUCTION
Influenza pandemics have occurred three times in the 20th century: in 1918, 1957 and 1968 [Reference Kilbourne1]. The impact of an influenza pandemic can be devastating. For example, it has been estimated that over 50 million people died during the pandemic of 1918 [Reference Taubenberger and Morens2]. Pre-pandemic planning, therefore, is essential if influenza pandemic-related morbidity, mortality, and social disruption are to be minimized. Avian influenza (H5N1) represents a serious threat of evolving into a human pandemic [3–Reference Webby and Webster5]. The possibility exists that the virus may mutate or adapt to allow efficient transmission in mammals or combine its gene segments with human influenza virus during co-infection of a single host resulting in a new and more lethal virus. A recent report that analysed a family cluster of H5N1 came to the conclusion that person-to-person transmission had probably occurred [Reference Ungchusak6]. Reducing the spread of influenza may be achieved by reducing the susceptibility of the population through vaccination or antiviral prophylaxis, by reducing the infectiousness of infected individuals by treatment and isolation, or by measures to reduce contact in the population. However, there are still little hard data to support these assumptions [Reference Bell7].
Due to the limited effectiveness of drugs in controlling the spread of a potential influenza pandemic, various non-pharmaceutical interventions (NPIs) are being proposed such as hand washing, face masks, school closure, social distancing or isolation, and travel restrictions. Mathematical models suggest that school closure during the peak of a pandemic can reduce peak attack rates by up to 40%. It may be that given enough drugs for 50% of the population are available, household-based prophylaxis coupled with reactive school closure could reduce clinical attack rates by 40–50% [Reference Germann8]. Other results based on simulations from the 1957–1958 Asian flu (attack rate 50%) suggest that social distancing such as school closure can mitigate the local progression of pandemic influenza without the use of vaccine or antiviral drugs by up to 90% [Reference Glass9]. Historical studies from the influenza pandemic of 1918 have shown that cities that quickly implemented NPIs such as school closure, cancellation of public gatherings and isolation, had greater delays in reaching peak mortality, lower peak mortality rates, and lower total mortality (~10–30%) than cities that were slow to implement these measures [Reference Bootsma and Ferguson10–Reference Markel13].
During an influenza outbreak, the highest attack rates occur in school-aged children and may exceed 30% [Reference King14]. There is a lack of understanding of the primary factors affecting the spread of influenza, and of population-based data [Reference Cliff and Haggett15]. Challenges include finding non-pharmacological methods to delay and flatten the peak of the case load.
During 2000, most primary public schools in Israel were closed as a result of a teachers' strike from 16 to 28 January. As a result, about 80% of all children between the ages of 6 and 12 years did not attend school. This offered a natural experiment to assess the effect of school closure on the spread of upper respiratory tract diseases including influenza. Using population-based data, we analysed the effect of the nationwide closure of elementary schools on the rate of ICD-9-coded medically attended respiratory-tract and influenza-like illness (ILI) as a surrogate for virus transmission in the population.
METHODS
Setting
This historical observational study was conducted in Israel in Maccabi Healthcare Services (MHS) and approved by the relevant research and ethics committees. MHS is a preferred-provider organization served by a nationwide network of >3000 physicians providing health-care services to 1·7 million members (24% of the country's population). Membership is open to all Israeli citizens and therefore representative of the general population. All Maccabi physicians use a computerized medical record exclusively for medical charting, and comprehensive and complete clinical data have been collected in a central database since 1995. Each patient has a unique and permanent identifier which is linked to all clinical and administrative data. These data include diagnosis at time of visit, all laboratory, imaging and pathology results, and a complete record of pharmacy purchases, hospitalization, outpatient and emergency department visits.
Analysis
The study period was from 1998 to 2002 inclusive. For each year we analysed three groups of individuals: school-aged children aged 6–12 years, household members aged >12 years presumed to be living with these children and all other Maccabi members aged >12 years. We found inconsistencies in the data for children aged <6 years and they were excluded from the study. Household members were grouped and linked to the identified head of household using our organizational database. These data are provided by the National Insurance Institute and confirmed by the members when they register in our clinics. Data on influenza virus prevalence was obtained from the Israel Centre for Disease Control of the Ministry of Health.
All clinic visit diagnoses related to any type of coryza-like and ILI. ICD-9 codes (460.0, 462.9, 463, 463.9, 464, 464.1, 464.11, 464.2, 464.21, 465, 465.9, 475, 478.9, 487.0, 487.1) were used, as it has previously been demonstrated that outpatient visits with influenza-like diagnoses can be attributed to the incidence of influenza in the community [Reference Cliff and Haggett15]. Prior to selecting these codes we performed a frequency analysis of all respiratory diagnostic codes reported in 2003 (n=463 354) and found that only 16 691 were for influenza compared to 231 562 for upper respiratory tract infection. Based on this analysis, we decided to use the above listed ICD-9 codes for coryza-like and ILI for our analyses. These codes will be referred to in this paper as ILI. The ICD group of diagnoses relating to respiratory illness other then our group of ‘ILI’ were excluded from the analysis. We determined the frequency of influenza-like and non-respiratory diagnoses for each of the 16 weekly periods from 5 December to 25 March, by year and by population group. We then calculated the ratio of the number of physician visits due to ILI to those for non-respiratory illness for each population group over each weekly period. We used this ratio rather than the absolute number of visits to minimize the effect of fluctuations in the absolute numbers of weekly visits and the annual increase in number of MHS members.
Statistical methods
Regression analysis was used to model the time dependence of the ratios (influenza-like to non-respiratory diagnoses) for each subgroup in each year. The regression models for the strike year included indicator variables representing the strike dates. The equation for the regression models is thus
where R t is the ratio in week t (t=1, …, 16), W 1 denotes the week number, W 2 and W 3 are variables that mark the weeks of change in the slope of the ratio with time, H is a variable that denotes the number of days of the Chanukah holiday in the preceding week, during which schools are closed for 8 days. (The dates of Chanukah vary from year to year, but typically are at the start of the influenza season in December.) S t is an indicator for the strike and εt is a random error term.
For the strike year (2000), S t equals 1 in the second week of the strike and the following week, as any effect on the spread of influenza would not be expected immediately. In each of the non-strike years (1998, 1999, 2001 and 2002), S t is synchronized to the annual influenza peak and equals 1 in the 2 weeks following the peak.
The regression model allowed estimating whether the strike had an effect on the ratio of the number of physician visits due to ILI to those for non-respiratory illness. To assess whether the strike year differed from the control years we fitted identical regression models to the latter, adding indicator variables (as if there had been a strike) in the 2 weeks following the peak of the ‘ILI’ season. The coefficient of the indicator variables estimates the change in the ratio associated with the timing of the strike. These changes were expressed as a percentage of the height of the estimated regression curve without the indicator, thus providing a measure of the change in the ratio relative to the estimated ratio, had there been no strike. Confidence intervals for the estimated percent changes in the ratio of the expected influenza-like/non-respiratory illness counts were computed from the regression results using the delta method. The effect of the strike on the school children was compared to all other effects (all other years and the other two subgroups in 2000) using Grubb's test (a standard method to test for an outlier from a set of data that have a normal distribution). Grubb's test is ideally suited to the goal of determining whether the effect of the strike on school children stands out from the other effects.
RESULTS
We examined information on all 21 932 000 physician visits recorded between 1998 and 2002. Of these 6 970 085 (31·7%) either had no diagnosis recorded or had non-diagnostic administrative ICD-9 codes only and were excluded from the analysis. Of the 14 961 915 visits with a valid diagnosis, the proportion of ILI was 12·6%. The annual proportions of ILI visits and total number of visits were 12·7% of 2·63 million (M), 13·3% (2·81M), 12·9% (3·13M), 12·2% (3·01M) and 11·8% (3·38M), respectively. The number of visits for each type of diagnosis and population group is presented in Table 1. The school closure (16–28 January 2000) corresponded with the peak number of influenza isolates from the Ministry of Health sentinel clinics which was during the weeks between 25 December 1999 and 29 January 2000.
Table 1. Number of physician visits due to influenza-like and non-respiratory illness, by population subgroup and year, Maccabi Healthcare Services, Israel, winter periods (5 December to 15 March, 1998–2002)
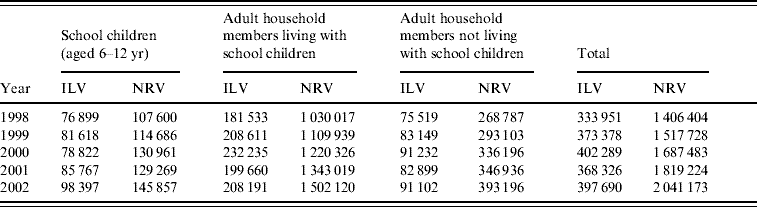
ILV, Influenza-like visits; NRV, non-respiratory visits.
The ratios of influenza-like to non-respiratory visits showed a similar pattern in each of the 5 years in each population subgroup. The data presented for each of the three population groups are juxtaposed so the peak times align (Fig. 1a–c). The 2 weeks of the strike are marked by two dots on the graphs. Over the 16-week period, the common pattern is that the ratio increases to a peak and then decreases. The rate of increase and the week of the peak changed from year to year.
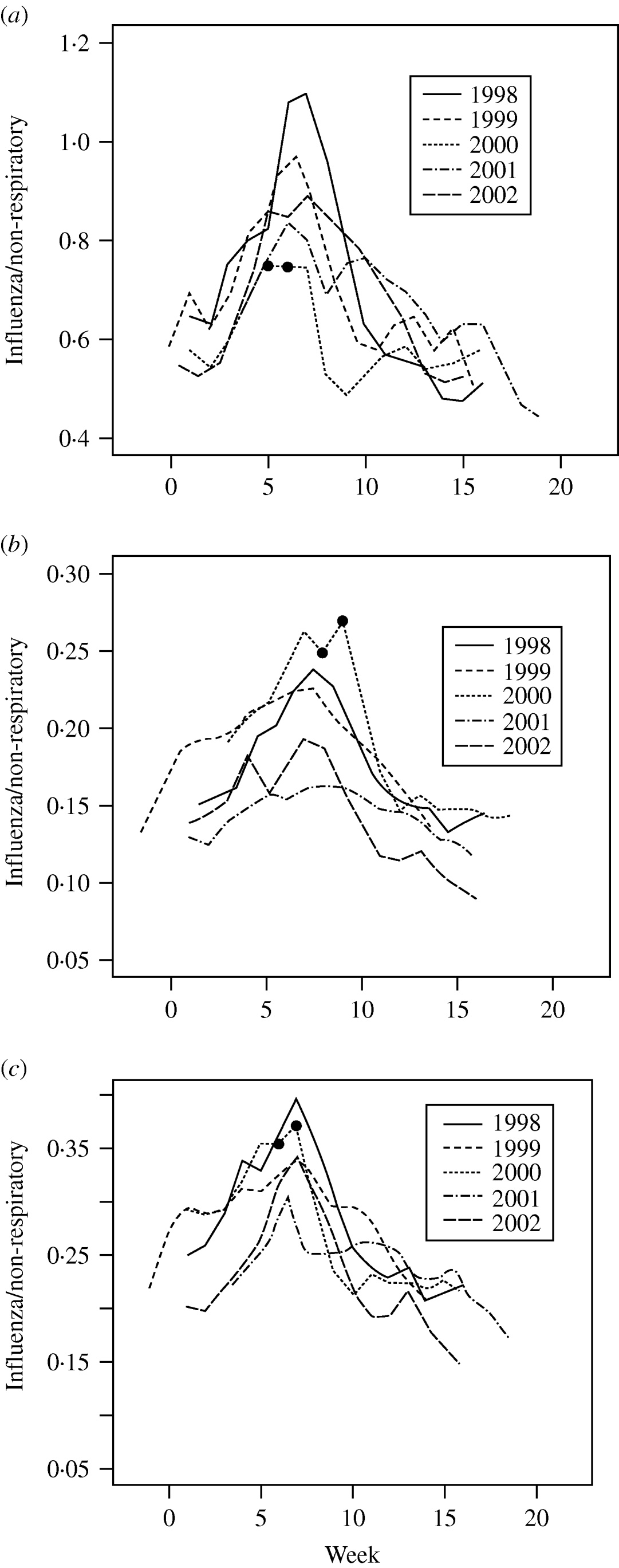
Fig. 1. The ratio of influenza-like to non-respiratory visits for (a) school-aged children (6–12 years), (b) individuals aged >12 years not living with school children (6–12 years), (c) individuals aged >12 years living with school children (6–12 years). The two small dots (•) mark the beginning and end of the strike. The weekly data presented are juxtaposed so the peak times align.
Separate regression models were fitted to each group in each year. All the regression models included: (i) a linear increase to the ‘peak’ ratio, (ii) a linear decrease from the peak ratio. In 1998, 2000 and 2002 (for all subgroups) and in 1999 (for school children only), the decrease in ratio near the end of the study period was much more gradual and a further slope change was included in the model.
We illustrate the definition of the variables W 2 and W 3 using the regression model for school-aged children in 1998. In that year, the slope of the ratio with time showed clear changes at weeks 7 and 10. The variable W 2 was set equal to 0 for weeks 1–7 and then equal to 1, 2, …, 9 for the remaining 9 weeks. The variable W 3 was set equal to 0 for weeks 1–10 and then equal to 1, 2, …, 6 for the remaining 6 weeks. The variable W 3 was included in the model only in the settings noted above where the slope changed near the end of the study period.
The 15 regression models for each of the three subgroups and each year fitted well to the data. Ten of the 15 regressions had R 2 values >94% and only two models were <90%. The strike variable significantly decreased the ratio for school-aged children in the strike year (P=0·007). A decrease was also seen for adults with no school-aged children in 1999 (P=0·037). The variable for the Chanukah holiday reduced the ratio for school-aged children in 1998, 1999 and 2001 (P=0·008, 0·006 and 0·045, respectively) and was statistically significant for both adult groups in 1999 and for adults with no school-aged children in 2001.
Table 2 shows the estimated change as a percent of the expected ratio from the model for all 5 years. We present in the table the effect of the strike summed over 2 weeks, the second week of the strike and the following week (for 2000) and the 2 weeks following the peak (for other years). In the strike year of 2000, the estimated decrease in the ratio for children aged 6–12 years is 14·7% for the 2 weeks most affected by the strike. The changes in the other years range from a decrease of 4·4% to an increase of 9·6% and differ sharply from the changes in the strike year.
Table 2. Estimated change (as a percent) in ratio of the expected influenza-like/non-respiratory illness, during the 2 weeks affected by the strike (in 2000) and the 2 weeks with similar proximity to the annual peak in the other years. Thus each year is treated as if there had been a strike affecting 2 weeks
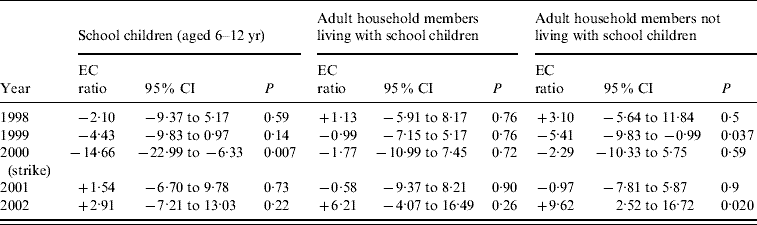
EC ratio, Estimated change (%) ratio.
P value is for the hypothesis of no change.
The steepest single-week drop in the ratios was also examined as an indicator of decreased ILI visits. For each group, the steepest drop occurs in the strike year, 2 weeks after the start of the strike.
The estimated changes in the ratio due to the strike (treating non-strike years as if there had been a strike) do reasonably follow the normality assumption, except for the change for school children in the strike year, which stands out. Grubb's statistic to check whether that result is an outlier was equal to 2·55, which is exactly the 5% critical value. So Grubb's test rejects, with P=0·05, the hypothesis that there was no effect of the strike on the flu/non-flu ratio for school children.
DISCUSSION
This study provides additional evidence of the impact of school closure on the spread of ILI in the community based on a comprehensive and extensive database of a country-wide health-care organization providing primary and secondary care to a large, stable population.
In this study, which is a natural experiment, we described the effect of school closure on ILI in children aged 6–12 years, their families and the surrounding community. We found that school closure had a significant effect on reducing the ratio of the number of physician visits due to ILI to those for non-respiratory illness for school children.
The steepest calculated drop in the ratio of ILI/non-respiratory illness in all three population groups occurred during the strike year compared to the two previous and the two following years. The effect was less marked in households with school-aged children than in households without school-aged children; this may be due to the presence of younger children in the same households who continued to attend pre-school day care. Although many parents were obliged to stay at home with their children during the school closure and thus may have been more available to consult with their physician, a marked decrease in all physician visits was noted. One could speculate that because a parent was at home, they were less anxious about their child's health and therefore delayed a physician visit or did not need to take the child to a physician in order to obtain an absence from work certificate due their child's illness. During the study period the main national vaccination effort was in the ⩾65 years age group and the influenza vaccination coverage in this group varied from 41% to 44%. The overall Maccabi population influenza vaccination coverage for the years 1998–2002 was about 10%. The vaccination coverage for children aged <12 years was <1%.
When a major antigenic shift occurs there will probably be insufficient time to produce and distribute a vaccine during the initial phase of the pandemic. In addition, even if an effective vaccine is developed in time, vaccination uptake may not be optimal [Reference King14]. As a result, greater emphasis will have to be placed on methods to reduce contact rate such as school closure, and antiviral prophylaxis [Reference Jefferson16]. Our results from this natural study suggest that school closure has an effect in reducing upper respiratory illness and support the mathematical models which suggest a modest effect of school closure as part of the NPIs that should be enacted in a pandemic. It should be noted that ultra-orthodox, preschool and high schools remained open during the study period. Had those schools been closed, the overall effect of the strike on ILI may have been augmented.
Limitations of the study
The main limitation of this study is the use of non-specific outcomes to define influenza. While clinical diagnosis of influenza in young healthy adults has been shown to be highly specific during periods of influenza circulation, it is much less so during other periods and in other populations such as the elderly, vaccinated persons and younger children [Reference Longini17, Reference Neuzil18]. The use of clinical diagnosis without viral isolates is particularly problematic in the 6–12 years age group who suffer from a variety of viruses that co-circulate [Reference Poehling19]. Thus, while this study clearly shows the effectiveness of school closure as an intervention for preventing seasonal respiratory viral spread throughout the community, it cannot be assumed that the same will be true for influenza. In addition, the design does not predict the magnitude of the preventive effect of school closure since it is assumed that an influenza pandemic will behave somewhat differently, both in the magnitude and speed of spread (and also perhaps the severity of the illness). Another limitation of this study is that some patients may have sought care in hospital emergency rooms and our diagnostic data were limited to clinic visits. However, the patients have same-day appointments in most areas of the country and do not have to rely on hospital emergency rooms. In this study we included only visits with clinical diagnoses. We believe this did not bias our results in a significant manner since the missing data mainly occurred in visits that were for administrative reasons which include repeat prescriptions. Over time physicians used a slightly lower proportion of administrative codes. However, there is no reason to suppose that coding changes are responsible for the reported effects. We did not include children aged <6 years in this study because many continued to attend preschool. Their presence in families with school-aged children may have weakened the apparent beneficial effect of the school strike. It may be that some children had ‘home’ congregate schooling during the strike, which may also have weakened the apparent effect of school closure. It may be that not all those identified as household members lived together.
In Maccabi 34% of the population are children and hence our results may not be applicable to Western countries with a lower proportion of children.
There have been a number of studies that point to excess number of physician visits by school-aged children during influenza epidemics [Reference Chiu20–Reference Principi22]. Children seem to be the main vector for disease transmission and are the major route of transmission of influenza viruses to household contacts [Reference Neuzil18, Reference Reichert23]. While children are rarely severely ill with influenza, they more frequently shed influenza virus than adults and for longer time periods [Reference Foy24]. The number of household members who become ill within 3 days of a child's absence from school was reportedly 2·2 times higher than expected during an influenza season and for every ten children who miss school for influenza-related illness, eight household members will subsequently become ill [Reference Neuzil, Hohlbein and Zhu25]. In a recent study of influenza transmission within households from an index case aged 5–15 years, clinical influenza was reported in 34% of the contacts. Of the 97 households in this study, 67 reported one secondary case, 26 reported two, and four reported three secondary cases [Reference Viboud26]. A recent study has shown herd immunity in adults against influenza-related illnesses with use of the trivalent attenuated influenza vaccine in children [Reference Piedra27]. An additional study that examined the effectiveness of school-based influenza vaccination by administering influenza vaccination in certain schools, found that households with children in the intervention schools reported significantly lower absenteeism both for children and their parents [Reference King14].
While in a previous study it has been found that school closure can limit the effect of an influenza outbreak in school children [Reference Heymann28], it was not clear whether limiting the incidence of influenza in school children will significantly affect the incidence in the general population. In the present study we have clearly demonstrated that school closure has the potential to significantly alter the effects of upper respiratory and ILI within a population comprising of both children and adults, and thus significantly reduce the burden of disease within the population. While we cannot be sure that school closure will mitigate the size of an epidemic, we can be confident, in view of the findings of the present study, that it will slow down its spread.
Measures such as the closing of schools or factories have been advocated as a way of reducing disease spread within a population during an influenza epidemic. Using a combination of strategies to contain the epidemic, such as delaying the spread of an outbreak throughout the population by closing all schools, while establishing chemoprophylaxis and immunization, may well be the optimal public health strategy available during the next pandemic.
ACKNOWLEDGEMENTS
We thank Professor Manfred Green, Director of the Israel Center for Disease Control, for his significant contribution to the content of this paper.
DECLARATION OF INTEREST
None.





