The discovery of Schmallenberg virus
In November 2011, a novel orthobunyavirus was detected in plasma samples from cattle with fever and reduced milk yield in a farm near the German town of Schmallenberg [Reference Hoffmann1]. The Schmallenberg virus (SBV) was traced using a metagenomic approach with next-generation sequencing resulting in seven short sequence fragments – out of more than 25 000 – with the highest homology to viruses of the Simbu serogroup of the genus Orthobunyavirus. SBV is most closely related to viruses of Sathuperi species such as Australian Douglas virus [Reference Yanase2, Reference Goller3]. The SBV infection represents the first known outbreak caused by a member of the Simbu serogroup in Europe.
First acute infections were detected in cattle in late summer 2011. They induced a short fever period and a marked reduction in milk yield in dairy cattle. In a number of farms, especially in The Netherlands, severe diarrhoea was a first marked clinical observation. In Germany, farms with robotic milking systems provided initial indications of a newly emerging health problem, as the daily collection of data on milk yield, body temperature and food uptake of individual animals allowed the detection of mild clinical signs occurring only in a limited number of cattle in a dairy herd (M. Holsteg, personal communication). Acute infections in sheep and goats in association with clinical signs had not been reported at the time when acute infections in adult cattle were observed, although there were some reports of diarrhoea of unknown cause in ewes, which were only communicated several months later. A short viraemia of only 5–6 days occurs during the acute phase of the infection in adult animals [Reference Hoffmann1].
Simbu viruses like Akabane virus are known to induce malformations if the embryo or foetus is transplacentally infected during a vulnerable period [Reference Parsonson and McPhee4]. Malformations due to SBV infection have been observed from December 2011 onwards in stillborn or newborn lambs, calves and goat kids, which were usually born at term. The first SBV-induced malformed lambs were detected in The Netherlands. The main pathological findings induced by SBV were identical to changes described for severe Akabane virus infections: arthrogryposis, torticollis, scoliosis and kyphosis, brachygnathia inferior and various malformations of the brain, cerebellum and spinal cord, including hydranencephaly and porencephaly [Reference van den Brom5, Reference Gariglinany6].
Spread of SBV epidemic
Examination of archived samples did not indicate the presence of SBV in Europe before 2011. The first evidence for transplacental transmission was obtained by examination of a calf that was bred in spring 2011 [Reference Hoffmann1]. It seems likely that this animal was infected early during the transmission season in 2011. First evidence for acute infections in adult cattle was obtained in August 2011. Therefore, introduction of the virus before 2011 and any earlier persistence of the virus in a hypothetical reservoir in Europe seems unlikely. The most plausible scenario is the first entry of SBV in spring or early summer 2011. All notified cases of malformed lambs, calves and goat kids that emerged from December 2011 onwards are the delayed consequence of the infection of pregnant sheep, cattle and goats which took place in summer or autumn 2011. Differences in the epidemic curves for different ruminant species are also influenced by seasonal breeding, in particular in sheep, and differences in the vulnerable periods during which transplacental infection may lead to foetal malformations in cattle, goats and sheep (Figs 1 and 2). Within a few months, the infection seems to have spread over a large area in Western Europe that included Belgium, France, Germany, Luxembourg, The Netherlands, the South and East of England and in 2012 also Switzerland. In the UK, a continuous survey of economic losses among farmers was performed, to obtain a better overview (http://www.defra.gov.uk/ahvla/2012/06/21/survey-measure-impact-schmallenberg-virus-sheep-farms/). In addition, sporadic infections were reported from Italy, Spain and Denmark (Table 1). It remains open whether this rapid spread has been exclusively caused by transmission through biting midges, in which SBV has been detected, or whether other modes of transmission, in particular other vectors, played a role.

Fig. 1. Cattle, sheep and goat holdings with Schmallenberg virus cases in Germany (as of 27 August 2012).
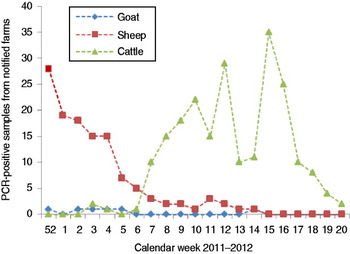
Fig. 2 [colour online]. Schmallenberg virus-positive detections (real-time RT–PCR of brain samples) in notified farms in The Netherlands (2011–2012).
Table 1. Confirmed cases of Schmallenberg virus infections in Europe

SBV seroprevalence
The first available information on SBV seroprevalence suggests that a large proportion of susceptible species (primarily ruminants) were exposed to the infection in the centre of the epidemic (>95% in North Rhine-Westphalia). In The Netherlands, the estimated seroprevalence of antibodies to SBV in dairy cows was 72·5% for cattle sera collected between November 2011 and February 2012. The seroprevalence of dairy cows in the central-eastern part of the country (83%) was significantly higher compared to the seroprevalence in dairy cows in the north (67%) and south (61%). High (70–100%) within-herd seroprevalences were observed in two SBV-infected sheep and dairy farms in which a considerable number of animals was tested [Reference Elbers7]. In areas with lower case counts in eastern and southern Germany, the seroprevalence was markedly lower (e.g. <10% in Mecklenburg-Western Pomerania; <20% in Bavaria) than in the centre of the epidemic. The serological data confirm the spatial distribution of SBV infections that occurred in 2011 as is evident from case detections by polymerase chain reaction (PCR). However, the data also show that a large proportion of sheep and cattle were exposed to SBV in the focus of the affected area, whereas the percentage of animals that had mounted an immune response to SBV was substantially lower in eastern and southern parts of Germany. If SBV-specific antibodies convey protection against re-infection, the level of immunity in the population will be high in The Netherlands and western parts of Germany, while a further spread of SBV in or from regions with a lower seroprevalence must be anticipated.
First serological surveys of wildlife in SBV-positive regions demonstrated a high number of SBV-antibody-positive red deer, roe deer and mouflon (H. Schirrmeier et al., unpublished results). Antibodies to SBV have also been detected in New World camelids (alpacas) [8]. Whether or not non-ruminant species like pigs and horses are susceptible to SBV remains to be clarified.
Role of vectors
Although vector transmission has not yet been formally established for SBV, several findings indicate that biting midges (Culicoides spp.) play a central role in the transmission of the disease. SBV has been detected in Culicoides spp. in Belgium [9], Denmark [Reference Rasmussen10], Italy, The Netherlands and Germany. In some cases, the infected insects could be typed as members of the Obsoletus complex or as C. dewulfi. The peak of transplacental transmission in sheep coincided with the highest risk of transmission of bluetongue virus serotype 8 (BTV-8) in Western Europe in 2006 and 2007 (Fig. 3), suggesting a very similar mode of transmission for SBV as for BTV-8. It is not clear, however, if other arthropod vectors can also transmit the infection. First experimental infections in cattle at the Friedrich-Loeffler-Institut and in sheep at the Central Veterinary Institute in Lelystad do not suggest that direct horizontal transmission plays a role in SBV transmission (Wernicke et al. and Van der Poel et al., unpublished data).
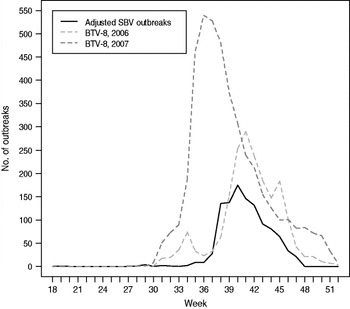
Fig. 3. Likely period of infection of pregnant sheep with Schmallenberg virus (SBV) in Germany in 2011 compared to case counts of BTV-8 in 2006 and 2007. Seventeen weeks were deducted from the reported date of birth of malformed lambs with confirmed SBV infection to calculate the most likely time of transplacental infection (assumptions: duration of pregnancy in sheep 150 days (range 145–155) days; risk period for SBV infection days 25–38 of pregnancy with the highest risk on day 32). Spearman's rank correlation: adjusted SBV outbreaks in sheep vs. BTV-8 in 2006: ρ = 0·894, P = 1·264 × 10−11; adjusted SBV outbreaks in sheep vs. BTV-8 in 2007: ρ = 0·857, P = 7·484 × 10−10.
Impact of the SBV outbreak
Economic losses due to SBV infections in livestock production can be considerable at the farm level. Within herds, the highest economic losses are observed in those sheep farms experiencing a high number of malformed lambs. Such malformations have been detected in about 4% of sheep farms and about 1·3% of cattle farms in the outbreak region in The Netherlands. In cattle farms, mostly single or a few cases of malformed SBV-infected calves were reported and only a relatively small number of goat farms were affected (Table 1). Economic loss in cattle due to delivery of malformed calves is limited and may be lower than losses due to milk yield reduction and return to service. However, to assess the impact of the SBV outbreak on animal production and animal welfare, more data is required as stated in a recent EFSA report [11]. It will be necessary to estimate the impact of the 2011 SBV infections on return to service, milk yields, rates of dystocia, congenital malformations and nervous symptoms in offspring. However, until now, data collection was only fragmentary; therefore more detailed studies will be needed to estimate the impact more accurately.
Nevertheless, SBV infections had caused substantial concern in farmers and the general public before any calculations of economic losses had been made. The emergence of the infection had a major impact on international trade of susceptible animals and animal products such as semen and embryos. More than 15 countries imposed restrictions on imports of live cattle from the European Union (EU). Additional restrictions on the import of embryos and semen of ruminants were, for example, imposed by the USA, Mexico and Japan.
However, based on the updated OIE factsheet on SBV [12], the EU is of the opinion that SBV does not deserve to be treated differently from Akabane virus, which causes a disease that is neither OIE listed nor notifiable in the EU nor subject to specific OIE standards or restrictions, although it is endemic in many areas of the world.
Public health aspects
After the SBV outbreak was established in December 2011, a first assessment of the potential human health hazard was made using a risk-profiling algorithm at the Dutch Institute for Public Health and the Environment (RIVM), by the German Robert Koch-Institut (RKI) and by the European Centre of Disease Control (ECDC) [13]. Since the risk for zoonotic transmission of SBV could not be excluded in the beginning, health complaints of potentially exposed persons were monitored. Serological studies were performed on humans, particularly in people living and/or working on SBV-affected farms. SBV-neutralizing antibodies were not detected in humans and it was concluded that there was no evidence for zoonotic transmission from either syndromic illness monitoring or serological testing [Reference Ducomble14, Reference Reusken15]. Therefore, the public health risk of SBV should be regarded as negligible.
Diagnosis
Diagnostic procedures for the detection of SBV infections became available very soon after the discovery of the virus and were rapidly distributed. They included (i) real-time reverse transcriptase (RT)–PCR (implemented and preliminary validation within days; validated commercial kits available after about 3 months [Reference Bilk16]), (ii) neutralization tests and indirect immunofluorescence assays (validated with the first virus isolate within a few weeks) and (iii) SBV antibody ELISAs allowing mass screening (available within about 5 months; a first commercial SBV antibody ELISA has been in use in several countries since May 2012 [17]). These techniques allowed the unambiguous diagnosis of SBV infections in malformed neonates by PCR or demonstration of pre-colostral antibodies with high sensitivity and specificity. The short viraemia limits the use of RT–PCR for the detection of SBV infections in adult animals to the acute phase of the infection. The sensitivity is highest in animals presenting with fever.
Measures taken
Immediately after the emergence of SBV was recognized, The Netherlands was the first country to impose mandatory notification of malformed calves, lambs and goat kids on test farms positive for SBV. It was thus possible to record accurately all infected farms. This measure was prompted by the fact that at the start a zoonotic potential could not be excluded and by the need to assess the impact of the epidemic rapidly. At a later stage, the disease was made notifiable in several other European countries including Germany and France. As a consequence, the number of notified cases (i.e. affected holdings in most countries) mainly reflects the distribution of SBV-induced malformations in neonates. Similar to the BTV-8 outbreak in 2006 and its re-emergence in 2007, the spread of SBV infections could only be recorded as there were no feasible measures to control the outbreak. However, the development of the epidemiological situation was swiftly communicated to trade partners and the general public.
For a vector-transmitted infectious disease, prompt detection and instigation of control measures such as vaccination are crucial to prevent spread. However, a vaccine is not yet available for SBV. Therefore, further spread of SBV can currently not be influenced by control measures directly aimed at the virus. However several institutes and companies are in the process of developing SBV vaccines, but the availability of licensed products before 2013 is unlikely. As for Akabane virus or BTV, inactivated virus preparations applied along with an adjuvant will be the first choice [Reference Kurogi18–Reference Eschbaumer20]. Later, depending upon the further spread and impact, cloned antigens as subunit preparations or recombinant modified live vaccines may offer substantial benefit for control. The most promising approaches are constructs targeting the replication within the insect vector, as was shown for Rift Valley Fever [Reference Bird21]. However, the development of a safe and efficacious vaccine including its registration for use is very time-consuming and expensive. Moreover, history has taught that it is extremely difficult to design a new reactive vaccination strategy swiftly. As a consequence, as in the early phase of other new epidemics, it is necessary to rely on biocontainment and biosecurity measures, which are not very effective for vector-transmitted diseases, as the fast and wide spread of SBV within a single transmission season has demonstrated markedly. Other possible control measures include changes in seasonal breeding which avoid the vulnerable period for transplacental transmission which falls in the vector-active period. However, such strategies may not be compatible with market demands.
Lessons to be learned from the SBV epidemic
First, the novel technology of metagenomics has proved to be very useful for the early detection of novel pathogens in livestock. SBV was detected during the acute phase of the epidemic and before the first malformed lambs and calves were born. As a result, diagnostic tools were available very early and could be used to follow the cases of SBV-induced malformation and to study, for example, seroprevalences. Veterinary diagnostics in Europe has proved to be prepared for this kind of outbreak situation and it has been shown that there is a very effective network of institutions working on epizootic diseases within the EU. This network has to be further supported, as was done in the past with the EU-funded Network of Excellence ‘EPIZONE’ (www.epizone-eu.net).
What to expect?
The rapid unnoticed spread of SBV in 2011 illustrates the risk of a further geographical spread of the infection in subsequent transmission seasons. However, the further dynamics of the infection depend also on the level of protection at the population level after exposure to SBV in 2011. As the role of potential vectors other than Culicoides spp. and the mechanisms of virus propagation in the arthropod vectors are still unknown, the future course of the epidemic is difficult to predict. Most likely, the infection will start again in those parts of SBV-affected regions where the seroprevalence is low, i.e. where a substantial proportion of the ruminant population is naive to SBV infection. In conclusion, a re-emergence and further spread of SBV can be expected in Europe. Furthermore, the spread of SBV by Culicoides spp. may be more efficient than the spread of BTV in the same region. Taking the Australian experience with Akabane virus into account, the spread of SBV to countries outside Europe may be possible. Only the combination of self-limitation and active vaccination will be able to interrupt further spread as seen with BTV-8 in Europe. However, since there is no vaccine available in 2012 the further spread of SBV in the next vector season cannot be actively influenced.
What to be prepared for?
SBV emerged in the same regions in 2011 as did BTV-8, BTV-6 and BTV-11 a couple of years ago. It thus seems that there is an unidentified ‘open door’ for exotic vector-transmitted diseases in middle Europe. The affected region has some unique features which may be of influence in this context: (i) several international airports, e.g. Amsterdam, Brussels, Cologne, and harbours such as in Rotterdam; (ii) a high human population density with the importation of large amounts of fresh goods, fruits, vegetables and flowers from throughout the world every day; (iii) a high density of cattle and sheep which represent a perfect target for exotic infectious diseases of ruminants; and finally (iv) domestic populations of Culicoides spp. which are competent for BTV, SBV and probably several other diseases transmitted by biting midges like epizootic haemorrhagic disease or African horse sickness virus.
In conclusion, further introductions of vector-borne diseases must be expected in this region. Jones and co-workers demonstrated that emerging infectious diseases are significantly correlated with environmental, socioeconomic and ecological factors and defined so-called ‘hot-spots’ for the introduction of infectious diseases into a new region [Reference Jones22]. This type of study can be the basis for identifying regions where new infectious diseases might emerge. Therefore, the combination of high densities of people and animals with high-frequency imports make North West Europe to a possible ‘hot-spot’ for the introduction of emerging infectious diseases. This should be taken into account when future surveillance, screening and sentinel programmes are planned. Fortunately, neither BTV nor SBV are zoonotic pathogens. However, related viruses like Oropouche virus or other vector-transmitted viruses like Rift Valley Fever virus might also be introduced and use the advantageous conditions described above which allow the fast and efficient spread of zoonotic agents.
What to do?
Strategies to improve veterinary and public health protection with regard to emerging pathogens have focused on improvements in monitoring and surveillance to allow the detection of new or unexpected diseases. Strengthening the concept of syndromic surveillance may be a possible approach to achieve this goal. It is important, however, to create monitoring and surveillance systems that do not ‘punish’ a policy of transparency and early warning in newly affected countries by unjustified trade restrictions imposed by countries deemed unaffected so far. Improved detection of viruses in reservoirs, early disease outbreak detection, or broadly based research to clarify important factors that favour (re-)emergence have to be supported and implemented. A main goal of infectious disease surveillance is early laboratory detection of newly emerging pathogens. Novel molecular methods, for example DNA microarrays and metagenomics offer unprecedented opportunities for rapid detection, but these require significant optimization and validation before they can be deployed broadly. In conclusion, the following measures are recommended based on the experiences with arthropod-borne (ARBO) virus outbreaks in Europe in recent years:
(1) A sentinel and vector monitoring programme should be installed in the regions with the highest risk for the introduction of vector-borne diseases (The Netherlands, Belgium, Western part of Germany). Sentinel herds (sheep, cattle, wild life) and vectors should be investigated on a regular basis.
(2) Novel technologies such as metagenomics with next-generation sequencing and microarray analysis have to be further optimized and updated, and should be used for the analysis of cases suspected of exotic infectious diseases.
(3) The awareness of farmers and veterinarians about the possible introduction of notifiable diseases like foot-and-mouth disease or African swine fever has to be raised and maintained at a high level. Easy access to differential diagnosis for notifiable epizootic diseases should be made available. The earlier a first sample is taken and analysed, the faster control measures can be implemented if necessary.
(4) National and international cooperation between institutes and cooperation between authorities should be improved as much as possible. In addition the ‘One Health’ approach, involving inclusive collaboration between physicians, veterinarians and other health and environmental professionals will be of increasing importance in combating emerging viral diseases.
Several research projects and networks about ARBO viruses like West Nile virus, Chikungunya virus or Crimean Congo haemorrhagic fever virus can also provide additional information [see e.g. the following selected project links: (www.arbo-zoo.net), (www.cch-fever.eu), (http://eurowestnile.isciii.es/ewn/default.aspx), (www.icres.eu), (www.vectorie.eu), (www.west-nile-shield-project.eu)].
ACKNOWLEDGMENTS
The scientific contributions of Dr Jörn Gethmann, Jana Sonnenburg, Dr Christoph Staubach, Dr Bernd Hoffmann, Dr Dirk Höper, Dr Kerstin Wernike and Dr Horst Schirrmeier as well as the technical support of Kathrin Teske and Doris Kämer are gratefully acknowledged. This work was partially funded by the European Union through the FP7 project ‘EMPERIE’ (contract no. 223498), and the Network of Excellence ‘EPIZONE’ (contract no. FOOD-CT-2006-016236), by the German Federal Ministry of Education and Research (Network of Competence of Agricultural and Nutritional Research ‘PHENOMICS’) and the Federal Ministry of Food, Agriculture and Consumer Protection.
DECLARATION OF INTEREST
None.






