INTRODUCTION
Salmonellosis is a foodborne disease that represents a major public health concern in both developing and developed countries [Reference Graham1]. The illness ranges from a self-limiting gastroenteritis by non-typhoidal Salmonella that usually resolves in 4–7 days [2], to a more protracted life- threatening typhoid fever [Reference Tamilarasu3].
Gastroenteritis caused by non-typhoidal Salmonella spp. is usually a self-limiting illness, but in 1–4% of patients, mainly the elderly and the immunosuppressed, bacteraemia may occur resulting in serious complications such as osteomyelitis, endocarditis or visceral abscesses [Reference Nãşcuþiu4]. In the United States, most foodborne illnesses are caused by norovirus (58%) and non-typhoidal Salmonella spp. (11%). Both agents are the leading cause of hospitalization (35%) and death (28%) in those with gastroenteritis [Reference Elaine5]. Majowicz et al. [Reference Majowicz6] estimated the international burden of non-typhoidal Salmonella gastroenteritis to be 93·8 million cases per year, resulting in 155 000 deaths. This reflects the enormous economic burden of the disease in both developing and developed countries [Reference Majowicz6]. The emergence of multidrug-resistant (MDR) Salmonella spp. to frequently used antibiotics tends to exaggerate the problem further. The rise in antimicrobial resistance has reduced the choice of effective treatment options and subsequently increased the treatment cost and risk of complications [Reference Tajbakhsh7].
The clinical illness caused by Salmonella enterica serovar Typhi (S. Typhi) infection manifests as typhoid fever, a major public health problem in developing countries, particularly in the Indian subcontinent and South East Asia [Reference Godfred8]. In endemic areas, the majority of cases occur in individuals aged <5 years. Clinical presentation varies from septicaemia in neonates, to diarrhoea in infants and to lower respiratory tract infections in older children [Reference Syed Ahmed9]. Typhoid fever is an endemic disease in Lebanon and the risk of outbreaks is still present [Reference Naji-Rammal10]. Antibiotics have a confirmed and beneficial role in the treatment of typhoid fever; however, their role in the management of non-typhoidal Salmonella gastroenteritis is questionable [Reference Onwuezobe11, Reference Sánchez-Vargas12]. The emergence and worldwide spread of MDR S. Typhi strains has led to an increase in morbidity, mortality and treatment failures [Reference Syed Ahmed9].
The aim of this article is to estimate the incidence of Salmonella infections in Lebanon in relation to age, gender, district and seasonal variation. The current Lebanese reporting system was assessed for its adequacy and reflection of the true burden of Salmonella illness in the country. The problem of antibiotic resistance in Salmonella isolates was addressed as well as the serious complications associated with salmonellosis.
METHODS
This is a descriptive epidemiological study based on records found at the Epidemiologic Surveillance Department at the Lebanese Ministry of Public Health (LMPH) between 2001 and 2013. Salmonella illness was defined as a patient with diarrhea, fever, and abdominal cramps and a positive fecal culture [2]. Cases were reviewed for the following information: annual incidence, age, gender, district and seasonal variation. Statistical analysis was done using the two-tailed t-test to determine if there was any statistically significant difference in the incidence between males and females. Results with a P value of ⩽0·05 were considered statistically significant. Since 2009 data related to S. Typhi were separated from non-Typhi as per the records of LMPH. An estimation of the annual incidence was done using the total yearly number reported divided by the estimated number of the population residing in the country. The burden of Salmonella illness was assessed through adjusting the reported incidence to account for the unreported cases using data from CDC, England and Jordan. A literature review of all published data from Lebanon about Salmonella susceptibility/resistance patterns and its serious clinical complications was conducted.
RESULTS
According to LMPH records, the average number of reported cases of Salmonella infection over the last 13 years was 611 (range 398–891) cases per year (Fig. 1). The estimated number of the Lebanese population residing in the country in 2013 was about 4·13 million [13], in addition to around 450 000 Palestinian refugees [14]. Thus, the estimated incidence of reported Salmonella cases per year was about 13·34 cases/100 000 individuals.

Fig. 1. Reported Salmonella cases per year 2001–2013.
Since 2009, the epidemiological surveillance department at LMPH started to differentiate between S. Typhi/Paratyphi and non-Typhi Salmonella spp. (Fig. 2). Their data revealed that the number of non-typhoid Salmonella cases reported is consistently lower than that of S. Typhi/Paratyphi.

Fig. 2. Reported Salmonella infections in Lebanon between 2009 and 2013 classified into Typhi and Paratyphi vs. non-Typhi.
The 20–39 years age group was most affected by S. Typhi and non-Typhi, with an average of 151 cases per year (around 25% of reported Salmonella cases). Infants and toddlers as well as those aged between 10 and 19 years were also commonly affected. The lowest incidence was seen in individuals aged 5–9 years and those aged >40 years (Fig. 3).
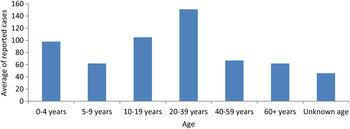
Fig. 3. Distribution of reported Salmonella cases in Lebanon according to age during 2001–2013.
There was no significant gender difference in the incidence of salmonellosis over the last 13 years (P = 0·834). The annual average number of reported Salmonella cases in males was 291 compared to 298 for females (Fig. 4).

Fig. 4. Distribution of reported Salmonella cases in Lebanon according to gender.
The distribution of cases along the six governorates of Lebanon is shown in Figure 5 a. The North had the largest number of cases (178 cases per year) followed by Bekaa (149 cases per year) then Mount Lebanon (122 cases per year), while the lowest was in Beirut (26 cases per year).

Fig. 5. (a) Percentage distribution of Salmonella cases along the six governorates of Lebanon between 2001 and 2013. (b) Percentage distribution of individuals residing in each of the six governorates of Lebanon [21].
Monthly distribution of reported Salmonella infections is shown in Figure 6. The peak incidence was in summer, mainly July and August (77 and 73 cases per year, respectively). The incidence decreases in autumn and winter and starts rising again in spring, peaking in the hot season.

Fig. 6. Seasonal distribution of Salmonella infection in Lebanon.
According to CDC it is estimated that for every one reported laboratory-confirmed Salmonella case, there are 29 unconfirmed cases [15]. Attempting to project this multiplier to the available Lebanese data will render the annual number of cases to be ⩾4/1000 individuals. Meanwhile, in Jordan it is believed that for each laboratory-confirmed Salmonella infection case there are about 273 infected persons in the community. If we use this multiplier which is the only one used to reflect the incidence in the Eastern Mediterranean Region (EMRO), the estimated annual incidence will be about 4/100 individuals [Reference Majowicz6, Reference Neyla16] in the absence of similar multipliers from Lebanon.
DISCUSSION
The estimated incidence of reported Salmonella infection in Lebanon is 13·34/100 000. It is lower than that reported by the CDC in the United States in 2012 (16·42/100 000) [17] and from Israel 30·3/100 000 in 2009 [Reference Bassal18]. This can be attributed to differences in the reporting systems and the adequacy of the surveillance measures in those countries compared to Lebanon. Reporting of Salmonella infections in Lebanon is based on clinicians’ and hospitals’ reports where patients with clinical symptoms and positive microbiology are reported. Clinical microbiology laboratories are not instructed by Lebanese health authorities to report their positive Salmonella results. Lower incidences were also reported from some neighbouring Arab countries with 2·3 and 13 cases/100 000 in Jordan and Egypt, respectively. The greatest burden of the illness is in India with 980 cases/100 000 [Reference Bradley and Schwartz19].
The surveillance system in Lebanon compared to that in the United States is less developed and less efficient and the Lebanese laboratories do not report their positive results to LMPH. This may indicate that the projected number derived from the CDC multiplier (4/1000) is an underestimate of the true incidence of the disease in Lebanon. As such, the reported cases merely represent the tip of a Salmonella infection pyramid. Similarly, data published from Jordan further support this concept where the discrepancy between confirmed cases and true incidence is explained by underreporting to the Ministry of Health and the suboptimal quality of laboratory specimen handling in relation to preservation and transportation [Reference Neyla16]. It is very unlikely that the completeness and ascertainment of laboratory-confirmed cases of Salmonella would be the same across all EMRO countries given the varying methods of surveillance and levels of socioeconomic development. The Jordan multiplier remains the closest conservative estimate of the Salmonella incidence in Lebanon. This verifies the need for establishing a local Lebanese multiplier. According to Wheeler et al. [Reference Wheeler20], in England the ratio of cases in the community to cases reaching national surveillance is 3·2:1. He described a surveillance pyramid and estimated loss of cases at different levels, where in every 1000 individuals 2·2 Salmonella cases are encountered in the community. Of these cases 1·6 present to general practice, 0·8 are detected by positive stool sample and 0·7 are reported to national surveillance. Due to the absence of similar information in Lebanon, this reporting pyramid cannot be implemented.
Of the six governorates of Lebanon, the highest reported numbers were from the North and Bekaa; yet, the highest population concentration is in Mount Lebanon (Fig. 5 b) [21]. This is not surprising since Bekaa and North are the most impoverished and deprived areas. Salmonellosis is a foodborne disease and its prevalence is associated with the standards of hygiene and public health infrastructure [22, Reference Foley23].
There was no significant gender variation in reported cases, similar to reports from the United States and Canada [24, Reference Csaba25]. However, the age distribution in Lebanon was different from that of the United States. According to CDC, Salmonella infections are most common in children aged <5 years [24]. This is in concordance with information published from the EMRO region where the higher incidence in those aged <5 years was attributed to several factors including a higher likelihood of being more exposed to infected items by mouth and their immune system which is still developing [Reference Bassal18]. The 20–39 years age group was that most commonly affected by Salmonella in Lebanon. This can be explained by the fact that salmonellosis is a foodborne disease with eating habits playing an important role in its transmission [2]. In a published report from Lebanon observing cases of typhoid fever between 2000 and 2008, drinking water was a less likely vehicle of transmission due to the fact that most Lebanese consume bottled water. However, water used for bathing, washing and cooking, remained a possible explanation since it was widely contaminated [Reference Kanj26]. According to PulseNet Lebanon, a foodborne diseases tracking network, 65% of 665 cases of food poisoning between 2011 and 2012 were attributed to Salmonella, and chicken and meat were the most frequent food product identified [27]. The very young and elderly population are less affected by Salmonella. This might be explained by the fact that they are less mobile than other adult categories. Besides being more housebound, they are more likely to consume bottled water and formula milk and escape the risks of eating outside home. Lebanon has been experiencing a nutritional transition in food choices during the past two decades from the typical Mediterranean home-prepared diet to the fast-food trend. The dietary habits of young adults are affected by the fast-food market [Reference Najat28]. The list of fast-food markets and restaurants violating health standards and regulations is increasing in Lebanon, necessitating an emergency and well publicized governmental initiative to maintain food safety [Reference Abu Faour29]. The results and outcome of this campaign are too early to assess.
Our data show seasonality in the occurrence of Salmonella with increased incidence in the warm season of July and August. This is similar to reports from CDC [24] and Saudi Arabia where 64·5% of Salmonella outbreaks occurred during the summer months [Reference Al-Goblan30].
Information about the serotypes of Salmonella isolates encountered and their antimicrobial susceptibility/resistance profiles are lacking from LMPH data. Between 2009 and 2013 more typhoid cases than non-typhoidal Salmonella cases were reported. This finding contradicts results published from a tertiary-care medical centre in Lebanon. Of all Salmonella isolates recovered between 2000 and 2011, Enteritidis and Typhimurium represented 18–44% and 12–34%, respectively, compared to Typhi (0–23%) [Reference Araj31]. In 2012, the CDC reported that Enteritidis (18%) and Typhimurium (13%) were the most common serotypes [17]. According to Somily et al., the main serogroups found in Saudi Arabia were D1 (24%), B (24%) and C (11%) [Reference Somily32], which include the serotypes, S. Enteritidis, S. Typhi (from group D1) and S. Typhimurium (group B). According to LMPH data, reported S. Typhi cases appear to have declined since 2010. There is no obvious reason for this decline and it is unclear whether the trend will continue.
Antibiotic susceptibility/resistant patterns are not included in the information collected from the epidemiology surveillance unit in LMPH's records. Published literature from Lebanon reveals that antibiotic resistance in Salmonella isolates is a major problem. According to Araj et al. [Reference Araj31], S. Typhi remained uniformly susceptible to third-generation cephalosporins, trimethoprim/sulfamethoxazole, fluoroquinolones and ampicillin until 2004. Since then, resistance to ampicillin and trimethoprim/sulfamethoxazole has begun to emerge, with susceptibility ranging from 65% to 100% to ampicillin, 43–100% to trimethoprim/sulfamethoxazole and no resistance to third-generation cephalosporins or fluoroquinolones being detected [Reference Araj31].
The situation regarding non-Typhi Salmonella [Reference Araj31] is different from that of S. Typhi, showing higher resistant rates to antimicrobial agents. Fluctuation in susceptibility to ampicillin ranged from 65% to 90% and to trimethoprim/sulfamethoxazole from 82% to 98% between 2000 and 2011 [Reference Araj31]. Compromised susceptibility to third-generation cephalosporins and fluoroquinolones has been detected since 2005 where susceptibility to cefotaxime was 88–100% and ciprofloxacin 91–100% [Reference Araj31]. Between 2008 and 2011 nalidixic acid resistance was detected, ranging between 25% and 57% [Reference Araj31]. Extended spectrum beta-lactamases producing Salmonella isolates were reported from Lebanon by Moubareck et al. in 2005 [Reference Moubareck33] and by Matar et al. in 2008 [Reference Matar34] and 2010 [Reference Matar35].
Salmonella spp. with reduced quinolone susceptibility has also been reported from the Middle East. Nalidixic acid resistance was 46% and 59% in Saudi Arabia and Palestine, respectively [Reference Somily32–Reference Al-Dawodi36]. Reduced susceptibility to ciprofloxacin in non-typhoidal Salmonella was also observed from Kuwait (14·2%) and the United Arab Emirates (7·4%) [Reference Vincent37]. A CDC report published in 2013 revealed that resistance or partial resistance to ciprofloxacin was 3% and 67% in Salmonella non-Typhi and Typhi isolates, respectively [38]. MDR S. Typhi is a major problem in the Indian subcontinent where many believe that what is observed merely represents part of a larger resistance problem [Reference Shrikala39]. As mentioned previously, antimicrobial resistance in S. Typhi isolates in Lebanon is still rare [Reference Kanj26] but the situation is different for non-Typhi Salmonella spp. where higher resistance rates to antimicrobial agents are encountered. Peculiar to Lebanon is the haphazard distribution and easy availability of antibiotics, lack of stewardship programmes and occasional lack of proper record keeping in Lebanese hospitals [Reference Michael40]. There is extensive consumption of broad-spectrum antibiotics such as third-generation cephalosporins and quinolones where more than 15% of the total national consumption consists of quinolones [Reference Michael40], an aspect that has been shown to be a major risk factor for the development of resistance [Reference Kanafani41]. In this respect, tracking antibiotic consumption and the emergence of resistant strains throughout the year may provide valuable information in the struggle against resistance.
Few case reports have been published from Lebanon describing the extra-gastrointestinal complications associated with salmonellosis. These complications were noted in both the immunocompetent and immunocompromised and were associated with variable clinical disease spectrum and serious consequences. These extraintestinal complications of salmonellosis are associated with more protracted treatment courses and poorer outcomes [Reference Jeffry42]. The reported complications of S. Typhi included rhabdomyolysis [Reference Dakdouki43], genital ulceration [Reference Omar44] and reversible encephalopathy [Reference El-Khoury45], while septic arthritis with bacteraemia complicated S. Enteritidis infection [Reference El-Herte46]. Reports from Israel suggested that bacteremia and urinary tract infections were the main extraintestinal manifestations [Reference Zaidenstein47]. In Spain, extraintestinal non-typhoid Salmonella serotype infections accounted for 1·8% of all salmonellosis cases and most of these infections occurred in children and the elderly [Reference Ruiz48]. Reports of focal infections like meningitis, abscesses, osteoarthritis and arterial infections caused by non-typhoid Salmonella have been published from the UK [Reference Ispahani49], where immunosuppression was the main risk factor [Reference Zaidenstein47]. Less frequently encountered complications of typhoid fever include splenic and sub-phrenic abscesses, pancreatitis, acute acalculus cholecystitis and wound infection [Reference Stauffer50–Reference Sfeir52].
CONCLUSION AND RECOMMENDATIONS
Salmonellosis is a major foodborne infectious disease with significant burden in both developed and developing countries. It is difficult to estimate the true burden of the illness in Lebanon and many Middle Eastern countries since available data lack crucial information and may not be fully representative. Despite their limitations, the existing information from the LMPH and the medical literature emphasizes the importance of salmonellosis in Lebanon and the problems associated with the infection, mainly of a socioeconomic and treatment-related nature. The emergence of antibiotic-resistant strains will complicate treatment and increase the cost of therapy. Despite the fact that those affected most in our study were aged between 20 and 39 years, it is the elderly, those with multiple chronic illnesses, the immunosuppressed, and those aged <5 years that remain at higher risk of serious complications if affected.
The epidemiological surveillance department at LMPH has to increase its surveillance efforts and start implementing mandatory periodic laboratory reporting of positive Salmonella cultures from various body fluids and sites. There is a need for a nationwide survey involving laboratories and general practitioners in order to estimate a multiplier more representative of the Lebanese situation. Information about antibiotic susceptibility/resistance patterns as well as Salmonella species must be included in the surveillance system. Antibiotic stewardship programmes are also urgently needed since their continued absence will only result in sustaining the increasing rates of resistance and an ever-increasing difficulty in the treatment.
The government has to engage in multiple campaigns to educate the public about food safety and the risk of foodborne diseases and maintain current efforts on food safety monitoring in order tyhat they become standard procedure. There is a marked need for increased alertness to halt any disease propagation in the region. This concern stems from the fact that both the country and the region are passing through a period of drought and decrease in rain levels [53]. Another compounding factor is the mass population displacement due to political instability and the current crises in Syria and Iraq which is spilling over to neighbouring countries.
DECLARATION OF INTEREST
None.









