Introduction
Vaccination against rubella began in Portugal in 1984, using a single antigen vaccine (R) recommended for girls aged 11–13 years [Reference Gonçalves1], in ‘a selective vaccination strategy’ that had already been used in other countries [Reference Robertson2, Reference Plotkin, Reef, Plotkin and Orenstein3]. Meanwhile, vaccination to prevent congenital rubella syndrome (CRS) evolved [Reference Palminha, Pité and Lopo4] to a ‘combined strategy’ [Reference Plotkin, Reef, Plotkin and Orenstein3] using two-dose schedules with a combined measles–mumps–rubella vaccine (MMR). The Wistar RA 27/3 strain was used in both vaccines (R and MMR) [Reference Gonçalves5].
In 1987, MMR was introduced into the PVP (Portuguese Vaccination Program), for both males and females, initially at 15 months of age [Reference Palminha, Pité and Lopo4, 6], followed by a second dose recommended for young adolescents in 1990 [7]. In 2000, the recommended age for the second dose of MMR was brought forward to 5–6 years [8] for those born after 1992 [Reference Palminha, Pité and Lopo4]. In 2012, the recommended age for the first dose of MMR was brought forward from 15 to 12 months of age [9]. Rubella vaccine was also recommended for susceptible women of childbearing age [7] and since 2000, adult women could be vaccinated with MMR, without previous serological evaluation, if they had never been vaccinated previously [Reference Saldanha and Azevedo10]. In 2012, the recommended age for the first dose of MMR was brought forward from 15 to 12 months of age [9]. Thus, there are two clear ‘vaccination generations’ of Portuguese women, depending on the official recommendations and available vaccines, which changed over time: those born before 1986 could not have been vaccinated as young children, while those born from 1986 had the opportunity to be vaccinated in childhood (with MMR); both generations might have received a second dose of vaccine against rubella (with MMR) later in life.
Data on the number of annual cases of rubella and CRS in Portugal, before and after vaccination was introduced, have limited validity and precision. Nevertheless, reports and serological studies point to a positive impact of vaccination against rubella in Portugal, both in serological/immunological terms and in the frequency of CRS [Reference Gonçalves1]. It has been shown that rubella virus transmission was interrupted in Portugal, leading to an elimination situation in the years 2012–2014 [11].
Immune responses to rubella vaccines are good, but the protective antibody levels reached are below those induced by the wild virus infection [Reference Best and Reef12]. Antibody concentration wanes with time after vaccination, but most vaccines remain immune [Reference Best and Reef12, Reference Cutts, Lessler and Metcal13]. Some seronegative individuals have shown secondary immune responses after revaccination with the rubella vaccine [Reference Cutts, Lessler and Metcal13]. Rubella antibody levels raised by booster vaccine doses tend to drop back to levels prior to revaccination [Reference Pebody14].
Good response to a single dose of vaccine against rubella (>95% seroconversion), associated with long-term persistence of protection, might not support the need for a second dose of vaccine [Reference Best and Reef12]. Nevertheless, countries that are able to sustain high vaccine coverage have been using combined MMR two-dose schedules, which seem to be useful for strategies for the elimination of both rubella and measles [Reference Cutts, Lessler and Metcal13].
One study from Iran, showed that the specific anti-rubella IgG (rIgG) concentration was lower in cord blood than in the corresponding maternal sera [Reference Doroudchi15]. Studies in several other settings have reported higher concentrations of rIgG in cord blood [Reference Gonçalves1, Reference Sato16, Reference Leineweber17]. Depending on the cut-off of seropositivity used, this shows that in some mother/newborn pairs, a woman may be classified as ‘susceptible’ while her newborn is ‘immune’ to rubella [Reference Gonçalves1].
In the 21st century, with the epidemiological situation evolving as a result of the strategies of vaccination against rubella, serological studies measuring rIgG in cord blood have been used to assess the immunological impact of vaccination in several developed countries, comparing rIgG levels of children from unvaccinated and vaccinated mothers [Reference Gonçalves1, Reference Waaijenborg Hahné18, Reference Takemoto19].
The present study aims to contribute to the evaluation of the serological impact of vaccination against rubella in Portugal, by measuring seropositivity in cord sera. Maternal blood samples were not obtained but, since rIgG in cord blood is of maternal origin, it was a proxy for the immune status of mothers. Since the efficient transplacental transfer of rIgG has been observed in Portugal [Reference Gonçalves1], we assumed that the proportion of susceptible mothers was equal to or superior to the proportion of susceptible newborns.
The specific objectives of this study were to measure the proportions of cord sera with rIgG levels corresponding to the negative or positive status (dependent variable), and to evaluate its association with the following potential predictive (independent variables):
• Sex of the newborn, because it is an important biological variable, and at least one study reported an association between sex and rIgG levels [Reference LeBaron20].
• Gestational age, because it has been shown to influence transplacental transport efficiency and thus, the levels of protective rIgG in newborns [Reference Gonçalves1, Reference Linder21].
• Maternal ‘vaccination generation’ (those born before 1986 or between 1986 and 1995), because it might be associated with the probability of having been exposed to the rubella wild virus, due to changes in the frequency of the natural infection over time [11].
• Maternal vaccination status, the main variable of interest (see text above).
• For newborns of vaccinated mothers, some specific aspects of their vaccination history were assessed as potential predictive variables such as time since the last dose, a number of doses received and maternal age at a first dose. The potential influence of these variables has been previously studied and discussed [Reference Best and Reef12].
Methods
This study used stored cord sera from a previous study [Reference Gonçalves22] conducted in the Obstetric service of a Portuguese NHS hospital, between October 2012 and March 2013. Approval for the study was obtained from the local primary health care board and the ethical committee of the hospital. After written informed consent was obtained, 206 mothers were interviewed. At birth, a cord blood sample was collected. Maternal blood samples were not collected. From the initial 206 samples, 198 sera were available for testing. Power calculations were not done as this was a convenience sample. We also had access to sera from a previous study together with reliable data on maternal vaccination status.
Individual maternal vaccination records were consulted during the interview whenever possible. Where those records were not available, the vaccination history was checked using computerised vaccination records in the primary care health centres. Thus, precise data on the number of doses and dates of vaccination against rubella were available.
Specific IgG antibodies to the rubella virus (rIgG) were measured in the sera, using the commercial immunoassay anti-rubella Virus ELISA (Euroimmun AG, Germany). Antibody levels were calculated and interpreted according to the manufacturer's instructions: rIgG levels below 8 international units per millilitre (IU/ml) were considered ‘negative’, those ⩾11 IU/ml ‘positive’ and those between 8 and 11 IU/ml results were considered ‘borderline’. As we were studying cord sera, which were likely to have higher concentrations of antibody than their corresponding maternal sera (not assessed in this study), we chose to interpret those with rIgG concentrations <11 IU/ml as ‘seronegative’. This is consistent with the rIgG cut-off levels used to consider people as protected against infection [Reference Best and Reef12] although this correlate of protection is not absolute owing to the role of cell-mediated immunity [Reference Best and Reef12]. Thus, the terms ‘susceptible/immune’ should be used with caution.
The general characteristics of the participants and some details of maternal vaccination histories were described. Potential predictive variables were dichotomised using the following cut-off values:
• Gestational age – the 37 week cut-off commonly used for the definition of preterm.
• Time since last vaccination – the median value of the continuous variable was used as cut-off.
• Vaccination status, vaccines used with the first dose and the age of 15 months to receive the first dose of vaccine – classes were chosen according to the objectives of this study, described above.
Analysis of the association between potential predictive variables and seronegativity/susceptibility (level of rIgG <11 IU/ml) was performed in three steps: among all (n = 198) participants, and in newborns from unvaccinated (n = 42) and vaccinated (n = 156) mothers. In each step, a classical univariate analysis was done, using 2 × 2 tables and χ 2 or Fisher's exact tests. After that, logistic regression models were used, fitting a final model after a backward stepwise approach. Variables remaining in the final models were significantly below the 5% level. Due to its distribution, variables like maternal age at first dose and number of vaccine doses received were not included in the logistic models and were only evaluated in specific birth cohorts. All statistical analyses were performed using SPSS®.
Results
General characteristics
Characteristics of the 198 newborns and their mothers are shown in Table 1. Most mothers (78.8%) had been vaccinated against rubella and they were younger than unvaccinated mothers in general (P < 0.0001). Only three mothers of the generation born between 1986 and 1995 (younger women), had never been vaccinated against rubella. The proportions of ‘positive’ and ‘negative’ cord sera were 92.4% and 7.6%, respectively.
Table 1. Characteristics of newborns and their mothers, and rubella IgG seronegativity in cord blood samples (n = 198)

a Data missing in four participants.
Maternal vaccination history
Some details on the vaccination history of the 156 vaccinated mothers are displayed in Table 2. Data are stratified by the maternal ‘vaccination generation’ if they had been born before 1986 (n = 119) or in 1986–1995 (n = 37).
Table 2. Data on vaccination against rubella of 156 mothers, by birth cohort

R, Monovalent vaccine against rubella; MMR, Combined vaccine against measles, mumps and rubella.
Women born before 1986 received both monovalent and MMR vaccines. Seven (6%) had received two vaccinations against rubella. For most of these women, vaccination began at much older ages than for those born between 1986 and 1995. None of these older women had been vaccinated against rubella before 15 months of age and most had received the vaccine after reaching 10 years of age.
For the generation born between 1986 and 1995, only MMR was used. This group began vaccination at much younger ages and ten of them were vaccinated before reaching 15 months of age. The two mothers receiving only one dose of MMR were the exception: they were vaccinated only after 10 years of age. As this was a much younger age group, the time elapsed since the last dose of vaccine was much less than for mothers born before 1986.
Determinants of the concentration of rIgG in the cord blood of all newborns (n = 198)
In 15 (7.6%) cord sera samples, the concentration of rIgG was below 11 IU/ml – classified as seronegative. The corresponding mothers could, therefore, have been susceptible to rubella. In addition, some mothers of newborns considered to be seropositive could also have been susceptible to rubella, although it was not possible to precisely estimate that number. As said before, the concepts of susceptibility/immunity are to be interpreted with caution. On univariate analysis, using 2 × 2 tables, showed that children from the ‘vaccination generation’ of mothers born between 1986 and 1995 were more likely (P = 0.056) to be seronegative. Being preterm and born to an unvaccinated mother appeared to increase the likelihood of being seronegative (at a significant level <0.10). However, after multiple logistic regression, the only predictive variables were vaccination status and ‘vaccination generation’ (Table 3). Newborns of mothers born between 1986 and 1995 (P = 0.010) and from unvaccinated mothers (P = 0.013) were more likely to be seronegative.
Table 3. Association between potentially predictive variables and seronegativity (rubella IgG <11 IU/ml in cord sera) in newborns (n = 198)
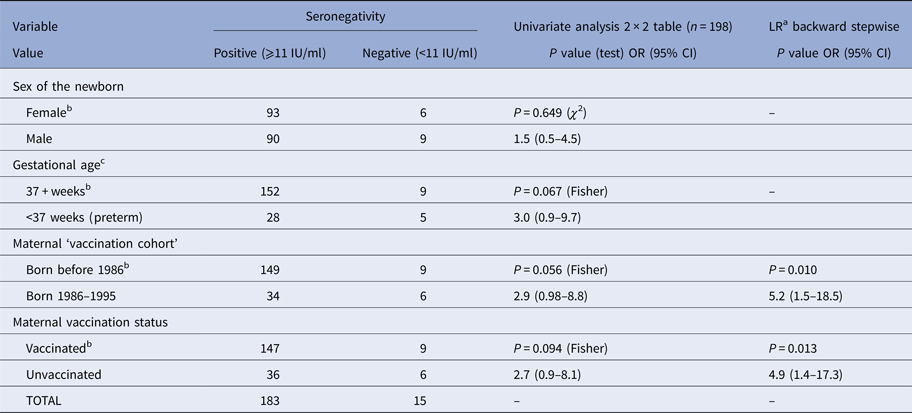
a Logistic regression (n = 194).
b Referent class.
c Four missing observations.
Determinants of the concentration of rIgG in the cord blood of newborns from unvaccinated (n = 42) and vaccinated (n = 156) mothers
In six (14.3%) of the newborns born to unvaccinated mothers (n = 42), the concentrations of rIgG in the cord sera were below 11 IU/ml (seronegative). None of the potential predictive variables was found to be associated with seronegativity.
In nine (5.8%) cord sera of newborns of vaccinated mothers (n = 156), the concentration of rIgG was below 11 IU/ml (seronegative). Both on univariate analysis and multiple logistic regression, the only significant predictive variable was ‘vaccination cohort’ (Table 4). Children of mothers vaccinated between 1996 and 1995 were more likely to be seronegative.
Table 4. Association between potentially predictive variables and seronegativity (rubella IgG <11 IU/ml in cord sera) in cord sera of newborns from vaccinated mothers (n = 156)
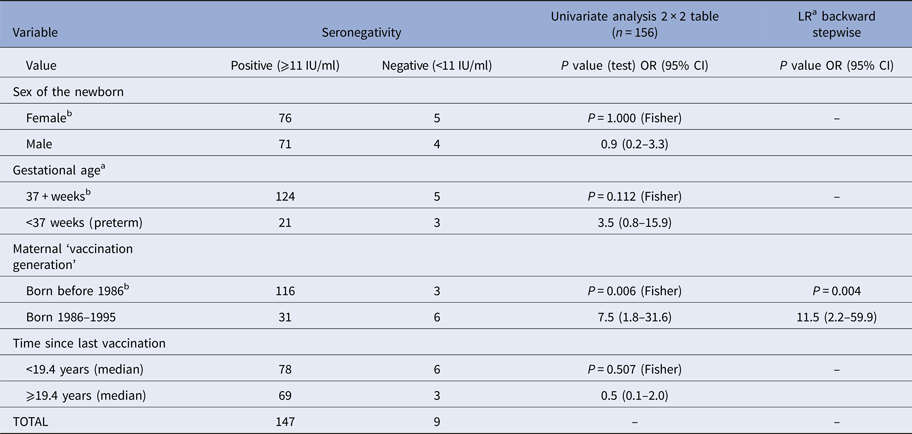
OR, odds ratio; LR, logistic regression.
a Three missing observations.
b Referent class.
The potential predictive value of the number of doses received (one or two) was analysed for vaccinated mothers born before 1986; the potential predictive value of receiving the first vaccine against rubella before the recommended age of 15 months was analysed for vaccinated mothers born between 1986 and 1995. In both cases, no association was observed.
Discussion
The main findings of this study were that:
(A) Most mothers (78.8%) had been vaccinated against rubella at least once in their lives and were younger than unvaccinated mothers. Among the 156 vaccinated mothers the type of vaccine, the number of doses and age at first dose varied according to age (having been born before 1986 or thereafter).
(B) For 15 (7.6%) cord sera samples, the concentration of rIgG was below 11 IU/ml, the newborns being classified as seronegative or susceptible. This is consistent with the rIgG cut-off levels used to consider people protected against infection [Reference Best and Reef12]. See comments below on the validity of the concepts ‘susceptible/immune’.
(C) Among all participants (n = 198), children born to unvaccinated mothers were more likely to be seronegative.
(D) Among all participants (n = 198) and children from vaccinated mothers (n = 156), children from mothers born before 1986 were more likely to be seropositive.
Internal and external validity
The data on maternal age and vaccination history were valid and precise. The main limitation of this study was that cord sera antibody seronegativity was used as a proxy for maternal immunity. However, as previously stated, the correlation between rIgG serum concentration [Reference Best and Reef12] and the concepts ‘susceptible/immune’ is not absolute and should be interpreted with caution. Therefore, it was possible that some ‘seronegative’ mothers were actually ‘protected’ against the infection. Nevertheless, as said in the introduction, other authors have used cord sera measurements [Reference Gonçalves1, Reference Waaijenborg Hahné18, Reference Takemoto19]. Commercial EIA assays have been recognised to be valid lab techniques to conduct seroepidemiological studies and the threshold for seronegativity used in this study has been recommended [Reference Best and Reef12]. Thus, the results reported here are both valid and comparable. While internal validity (association between variables) can be trusted, extrapolations to the entire Portuguese population and to other populations should be made cautiously.
Comparison with results from other studies
The proportion of vaccinated mothers (78.8%) in the present study (findings ‘A’) is much higher than the 52.8% coverage observed in Portuguese mothers giving birth in 1993/1994 [Reference Gonçalves1], the value of 65.6% reported among Dutch mothers in 2006–2007 [Reference Waaijenborg Hahné18] and the 38% coverage in Japanese mothers in 2013 [Reference Takemoto19]. Among participants in this study, the type of vaccines used and ages of administration varied with maternal age, which is consistent with the evolving changes in the Portuguese vaccination schedule, as reported in the ‘Introduction’.
It was likely that the mothers of the 15 (7.6%) seronegative newborns (findings ‘B’) had been susceptible to rubella during pregnancy and it was also likely that a small proportion of mothers of seropositive newborns were also susceptible, although it was not possible to precisely estimate that proportion. The finding ‘C’, that the proportion seronegative was higher among newborns from unvaccinated mothers is not consistent with findings from other studies but could mean that rubella wild virus has not been circulating in Portugal for a long time now and some unvaccinated women might not have come into contact with the wild virus. In a previous Portuguese study conducted among mothers giving birth in 1993/1994, seronegative cord sera were also more frequent among children from unvaccinated than from vaccinated mothers (P = 0.008); however, the results are difficult to compare, because a different commercial lab technique and a higher concentration cut-off value were used to measure antibodies and classify them as seropositive/seronegative; in any case, that study identified more seronegative (susceptible) mothers than newborns [Reference Gonçalves1]. In a Dutch study, the lab technique used was different, making comparisons with the present study practically impossible; nevertheless, there were small (non-significant) differences in rIgG concentration between vaccinated (general population) and unvaccinated mothers (from a religious minority group) [Reference Waaijenborg Hahné18]. In a Japanese study conducted in 2013 [Reference Takemoto19] 5.7% of cord sera were seronegative and although the precise seronegative proportions among newborns from vaccinated and unvaccinated mothers were not reported, the concentration of rIgG was higher among those born from unvaccinated mothers, who were assumed to have been infected with the wild virus; again, comparisons with the present study are made difficult because of the different lab techniques and cut-off values.
Our finding that time since last maternal vaccination was not associated with the likelihood of newborns being seronegative is consistent with the previous knowledge that although antibody concentration wanes with time after vaccination, most vaccinees remain immune for life [Reference Best and Reef12, Reference Cutts, Lessler and Metcal13].
The observed lower proportion of seronegative newborns from mothers born before 1986 is consistent with previous theoretical knowledge and changing epidemiological situations. Immune responses to rubella vaccines are below those induced by the wild virus infection [Reference Best and Reef12]. On the other hand, although we have no precise data, it is expected that Portuguese women born before 1986 were more likely to have been infected with the wild rubella virus [11]. These types of findings and explanations were also described in a Japanese study [Reference Takemoto19].
Recommendations on vaccinating women in childbearing age
From the findings of the present study, it was observed that some vaccinated and some unvaccinated mothers were susceptible to rubella such that there was a hypothetical risk of CRS. Those situations were rare but, nevertheless, undesirable and potentially preventable. Thus, the vaccination status of women of childbearing age should be checked. Since 2000, the Portuguese guidelines have stated that adult women can be vaccinated with MMR without previous serological evaluation if they had never been vaccinated previously [Reference Saldanha and Azevedo10].
One of us (JF) contacted the 15 mothers of seronegative children by telephone, advising them to discuss the result with their General Practitioner. We hope that this may have helped to protect future pregnancies from the risk of CRS.
Acknowledgements
Commercial ELISA Kits were purchased by the Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto, and Escola Superior de Saúde do Instituto Politécnico de Leiria, Portugal. The authors are grateful for the support given by several health professionals. Dr Helder Roque and Dr Alicia Rita authorised the study in the Obstetric Service of the hospital of Leiria, Portugal. Dr Jorge Costa authorised the consultation of vaccination records in Leiria. Nurses Leonor Silva, Cesaltina Sousa, Fátima Soares, Dina Pascoal, Susana Frade and their teams helped with the collection of cord blood samples and in the consultation of vaccination records. We are grateful to Professor Leonie Prasad for her careful revision of the text. The 198 newborns and their mothers are also gratefully acknowledged.
Conflict of interest
None.






