Introduction
Soil-transmitted helminthiases (STH), such as the one caused by Ascaris lumbricoides, are the most prevalent parasitic infection globally. Recent estimates from the 2019 Global Burden of Disease Study indicate that around 446 million people worldwide are infected with A. lumbricoides, with an estimated disease burden of 754 000 Disability Adjusted Life Years (DALY) [1]. Moreover, 1.5 billion people worldwide are estimated to be affected by STHs, accounting for an estimated global disease burden of 1.5 million DALY [1]. Additionally, 5.9 billion people worldwide are estimated to be at risk of acquiring a STH [Reference Pullan and Brooker2, Reference Pullan3].
A. lumbricoides is an anthroponotic intestinal helminth acquired via the faecal-oral route. Adult male and female worms live and copulate in the human intestinal lumen where female worms lay eggs that are released into the environment with the faeces. Unfertilised eggs can also be laid in absence of copulation, but these eggs are not infective. Fertilised eggs need to mature in the environment to embryonate and become infective, which takes from 10 to 50 days, depending on the specific environmental conditions [Reference Walker, Hall, Basáñez and Holland4]. Infection happens when a human host ingests embryonated eggs through soil-contaminated food or water, as well as through ingestion of eggs on contaminated fomites, body parts (e.g. dirty hands) and the soil itself. Once ingested, eggs hatch in the intestine and the ensuing larvae penetrate the gut wall, migrate via the hepatic-portal blood to the heart and lungs, ascend through the trachea and larynx, and are eventually swallowed back to the intestine where they mature to adults and start laying eggs. This entire pre-patent period usually lasts 9–11 weeks [Reference Bethony5].
Anthelmintic treatment is one of the most common methods to reduce STH prevalence at the community level, with preventive chemotherapy based on anthelmintic mass administration to pre-schoolers (24–59 months of age) and school-aged children (5–12 years of age) often used as the primary intervention to reduce morbidity, as advised by the World Health Organization (WHO) [6–9]. However, to interrupt STH transmission, anthelmintic administration to adults seems necessary, for a period of at least ten years and eventually establish complementary interventions, such as provision and usage of WASH (water, sanitation and hygiene) resources [Reference Anderson, Truscott and Hollingsworth10–Reference Pullan14].
Optimal use of anthelmintics against A. lumbricoides in endemically infected communities is challenged by several host-related and environmental factors influencing the risk of acquiring A. lumbricoides infection after treatment, which vary across cohorts. The type, dosage, coverage, effectiveness and frequency of administration of anthelmintic drugs, as well as several demographic characteristics (e.g. age, gender, occupation, education, financial income, family structure, geographical location, social class, etc.) of the affected individuals and their households, are some of the factors to consider [Reference Anderson, Truscott and Hollingsworth10, Reference Forrester15–Reference Jia21]. STH control in Venezuela has had a history of success for the last six decades, with A. lumbricoides prevalence being reduced from nearly 60% in the 1940s and 1950s to 27% in the early 1990s, and to 5% more recently (2007–2010). The latter prevalence estimate concerns urban areas and might be partly due to the STH control programmes, as well as changing living conditions, whereas in rural areas prevalence can still be as high as 40%. In the last two decades, very limited and irregular actions have been taken to administer anthelmintic drugs in schools [Reference Incani22]. In 2019, an attempt at preventive chemotherapy using mass drug administration was made following WHO recommendations, administering 4 million doses of albendazole to both pre-schoolers and school-aged children in urban and rural areas. Another attempt was performed in 2021 on a comparable number of children, both in rural and urban areas, covering between 60 and 80% of the target population. However, no coprological testing could be performed, neither pre- nor post-treatment, to assess the impact of the intervention. At present, Venezuela is listed by the WHO as having a coverage of mass drug administration among children of 75% or more, for a period of less than five years [8].
Here, we present the results of a longitudinal field study in which we assessed the risk of acquiring A. lumbricoides infection after anthelmintic treatment in an endemically infected rural community in Venezuela. This study was performed to assess specifically: (1) how long it takes for A. lumbricoides infection levels in the community to build up again after treatment, (2) which groups of people are more likely to acquire the infection, and (3) at which rate they acquire the infection. The results of this study are expected to provide guidance to mass drug administration strategies for ascariasis in this type of resource-limited settings where the prospects of routinely assessing STH control interventions to identify opportunities for improvement are yet to be implemented.
Methods
Study community and ethical clearance
The study was conducted in ‘Caserío El 25’, a rural community located in the Carabobo state, Venezuela. A detailed description of this community can be found elsewhere [Reference Incani, Grillet and Mughini-Gras23]. This study was part of a larger research project aimed at elucidating the epidemiology of intestinal parasite infections in Venezuela's rural communities. The objectives of the project were explained to the members of each household in the study community to obtain written informed consent from all adults and parents or legal guardians of children (<18 years old). The study adhered to local ethical criteria and was approved by the Ethical Committee of the Carabobo State Health Authority (INSALUD), Venezuela, and by the Ethical Committee of the VU University of Amsterdam, the Netherlands. The study design was longitudinal and involved the investigation of factors influencing the risk of A. lumbricoides (re)infection. Anthelmintic treatment (pyrantel) was given to the study participants to define the start of the at-risk period for the time to (re)infection. At the time of the study (December 2008–March 2010), the community was composed of 470 inhabitants living in 85 houses. The whole community was invited to participate and 224 people across 55 houses consented and were enrolled into the study.
Sampling procedures
In total, six consecutive samples of faecal material were obtained from each participant. The first sample was collected pre-treatment (‘baseline’ sampling), and the subsequent five samples were collected at 1, 3, 6, 9 and 15 months post-treatment. Each sampling lasted a maximum of three days, meaning that participants provided the samples within a temporal window of three days.
Treatment
Pyrantel was administered as a single oral dose with a suspension of 10 mg/kg, two weeks after the baseline sampling. Fourteen participants in the baseline sampling could not be treated because they were absent from home when the treatment was administered. These participants were therefore excluded from further sampling. At the end of the study (i.e. after the sampling at 15 months post-treatment), all participants were offered (free of charge) a single dose of pyrantel as a deworming agent. Pyrantel as a paralyzing anthelminthic drug was used instead of albendazole because this study was part of a larger study that also included adult A. lumbricoides worm expulsion.
Collection and processing of faecal samples
Faecal samples were collected individually in 10 ml faecal collectors, kept refrigerated and transported to the laboratory at the University of Carabobo in Valencia, Venezuela, the same day of collection. At the laboratory, 0.15–0.50 g of each sample was added to a pre-weighed 7 ml rubber stoppered VacutainerR tube with 4.5 ml merthiolate-iodine-formaldehyde (MIF) fixative in an electronic scale with a sensitivity of 1/10 of a mg. Timing between faeces collection and fixation in the laboratory was between 6 and 8 h. Tubes were then reweighed after adding the faeces to obtain the amount of faeces examined from each participant. After vortexing the tubes for 5 s, 100 μl of faecal suspension was transferred onto microscopy slides, covered with 24 × 50 mm coverslips, and sealed with melted paraffin to avoid desiccation. Two samples of faecal suspension per tube (i.e. per participant) were thoroughly examined at the microscope to count A. lumbricoides eggs and to estimate the number of eggs per gram (EPG) of faeces. A third slide was examined when the difference between two counts was more than 20% [Reference Sapero and Lawless24, Reference Incani25]. While MIF and Kato-Katz techniques show good agreement when two MIF slides are examined [Reference Incani25], MIF was preferred over Kato-Katz because it eliminates the urgency for sample examination that Kato-Katz requires and, therefore, MIF is preferred when processing large numbers of samples in a short period. Additionally, MIF allows for re-examination of samples and easier detection of high parasitic loads, while Kato-Katz egg count at high parasitic loads becomes difficult. Kato-Katz has been used in the same faecal samples to compare its performance with MIF and the results have been reported previously [Reference Incani25].
Data analysis
Prevalence and mean intensity of infection (i.e. EPG among the positive samples) were calculated at each sampling period. Potential effects of participants' sex and age before treatment (≤5, 6–10, 11–15, 16–25, 26–45, ≥46 years) were assessed over sampling periods. Moreover, previous studies in the same area identified a number of factors associated with STH infection risk referring to socio-economic conditions [Reference Incani, Grillet and Mughini-Gras23]. Therefore, a variable indicating the socio-economic status (SES) of the households was defined using the Graffar scale [Reference Graffar26], which measures SES based on five factors: the occupation of the ‘head of the household’ (i.e. the person with the highest level of occupation), maternal educational attainment, household income, living conditions in the home and state of the neighbourhood of residence. The scale consists of five classes and all households in this study fell within the two lowest classes: lower-middle SES (class IV) and lower SES (class V). Potential effects of SES on A. lumbricoides prevalence and intensity of infection were also assessed over sampling periods and statistical analysis was performed using multivariable generalised linear mixed-effects models (GLMMs) with a logit link and binomial error distribution (for dichotomous data, i.e. positive/negative samples), or a log link function and negative binomial error distribution (for count data, i.e. EPGs). The GLMMs included two nested random effects to account for repeated measurements from the same participants over time, as well as clustering of different participants living in the same households.
Survival rates were estimated using the Kaplan-Meier method and survival curves were compared using the log-rank test. Nelson-Aalen cumulative hazard functions were also used for visualisation of the cumulative number of expected infections. Multivariable Cox proportional hazards regression models for multiple-record-per-subject data, with time-since-treatment (i.e. 1, 3, 6, 9, or 15 months) as the time-scale and entry into the at-risk period after the treatment, was then used to calculate hazard ratios (HR) and 95% confidence intervals (95% CI) for infection. Covariates included in the Cox model were the age at treatment, sex, SES, and presence/absence of the infection before treatment. Non-significant covariates were removed using backward elimination. A cluster-robust sandwich variance estimator was used to account for the clustering of participants at the household level. All analyses were performed using Stata 16 (StataCorp, College Station, TX, USA). Overall, a P < 0.05 was considered as statistically significant.
Results
Prevalence of A. lumbricoides infection
Summary statistics about A. lumbricoides infection prevalence are given in Table 1. The cohort comprised 224 participants living in 55 households, consisting of 44% males and 56% females, with a median age of 13 years (mean 21 years, interquartile range (IQR) 1–72 years). Overall A. lumbricoides infection prevalence was 39.7% (95% CI 29.0–51.5%) at baseline. However, over the whole period of sampling, 123 (55%) participants had at least one sample positive for A. lumbricoides.
Table 1. Prevalence of A. lumbricoides in the study population

a Total number of samples tested.
b 95% confidence interval within parentheses is adjusted for clustering of participants at the household level.
c OR, Odds ratio, the 95% confidence interval is shown within parentheses. Estimates are adjusted for all variables included in the table. Two nested random effects were included in the model to account for repeated measurements from the same participants over time and clustering of different participants living in the same households.
GLMM analysis revealed that A. lumbricoides infection was significantly less prevalent in all age groups above 16 years as compared to children under 5 years of age (16–25 years: Odds Ratio (OR) 0.36, 95% CI 0.14–0.92, P = 0.034; 26–45 years: OR 0.13, 95% CI 0.05–0.30, P < 0.001; ≥46 years: OR 0.06, 95% CI 0.02–0.18, P < 0.001). Prevalence was also significantly lower in females than in males (OR 0.60, 95% CI 0.37–0.99, P = 0.047), and at all sampling periods post-treatment as compared to the baseline (1 month: OR 0.03, 95% CI 0.01–0.06, P < 0.001; 3 months: OR 0.14, 95% CI 0.08–0.27, P < 0.001; 6 months: OR 0.46, 95% CI 0.26–0.81, P = 0.007; 9 months: OR 0.42, 95% CI 0.24–0.74, P = 0.003; 15 months: OR 0.50, 95% CI 0.29–0.88, P = 0.016), but prevalence was significantly higher in people living in households of lower as opposed to lower-middle SES (OR 3.67, 95% CI 1.27–10.59, P = 0.016) (Table 1).
Considering the first two samplings (pre-treatment and 1 month post-treatment), 86.2% of the participants that were positive for A. lumbricoides before treatment became negative the first month post-treatment.
Intensity of infection
Table 2 shows the mean intensities of infection (i.e. EPGs) among the positive samples. At baseline, mean EPGs were 30 096 (standard error 5092) for A. lumbricoides (89 samples). GLMMs showed that mean EPGs were significantly lower among participants aged 11 years or older as compared to children under 5 years of age (11–15 years: Incidence Rate Ratio (IRR) 0.44, 95% CI 0.24–0.82, P = 0.010; 16–25 years: IRR 0.24, 95% CI 0.11–0.52, P < 0.001; 26–45 years: IRR 0.18, 95% CI 0.09–0.37, P < 0.001; ≥46 years: 0.27, 95% CI 0.09–0.77, P = 0.014). Mean EPGs were also significantly lower among samples collected at 1–6 months post-treatment as compared to before treatment (1 month: IRR 0.18, 95% CI 0.09–0.38, P < 0.001; 3 months: IRR 0.27, 95% CI 0.16–0.46, P < 0.001; 6 months: IRR 0.60, 95% CI 0.38–0.92, P = 0.020), but not at 9 or 15 months after treatment. Moreover, mean EPGs were significantly higher in people living in households of lower compared to lower-middle SES (IRR 1.87, 95% CI 1.03–3.38, P = 0.039) (Table 2).
Table 2. Mean intensity of infection (eggs per gram of faeces – EPG) for A. lumbricoides in the study population
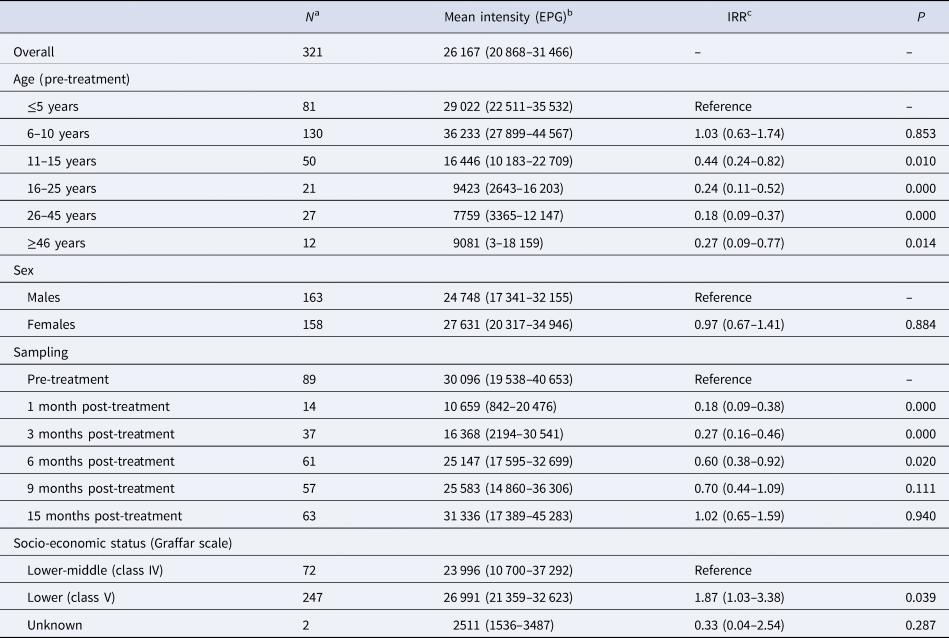
a Total number of positive samples.
b Calculated based on positive stool samples only, 95% confidence interval within parentheses adjusted for clustering of participants at the household level.
c IRR, Incidence rate ratio, the 95% confidence interval is shown within parentheses. Estimates are adjusted for all variables included in the table and for clustering of participants at the household level. Two nested random effects were included in the model to account for repeated measurements from the same participants over time and clustering of different participants living in the same households.
Survival analysis
Survival analysis for the time to (re)infection after treatment was based on 210 participants that received treatment (i.e. 14 participants could not be treated because they were absent from home at the time of treatment and were therefore excluded from further analyses). The included participants accounted for a total of 2357 person-months at risk during which 90 infections were observed, resulting in an overall incidence of 3.8 A. lumbricoides infections per 100 person-months (95% CI 3.1–4.7). Median time from treatment to infection was 6 months (mean 6 months, IQR 3–9): at 1, 3, 6, 9 and 15 months post-treatment, the cumulative incidence of A. lumbricoides infection was 6.7%, 18.9%, 34.6%, 42.2%, and 52.6%, respectively (Fig. 1). The median age at A. lumbricoides infection after treatment was 8 years (mean 13 years, IQR 5–13 years). Survival curves for A. lumbricoides infection differed significantly depending on age, SES, and prior (i.e. pre-treatment) A. lumbricoides infection (Fig. 2), but not sex. The Cox proportional hazards model for A. lumbricoides infection (Table 3) also showed that the HRs in all age groups ≥16 years were significantly lower than in children ≤5 years. Moreover, those who had an A. lumbricoides infection before treatment (compared to those who did not have this infection before treatment) were at increased risk of being (still or again) positive for A. lumbricoides after the treatment. The same was true for people living in households of lower SES. No significant differences were observed for sex (Table 3).

Fig. 1. Nelson-Aalen cumulative incidence of A. lumbricoides infections over time since anthelmintic treatment.
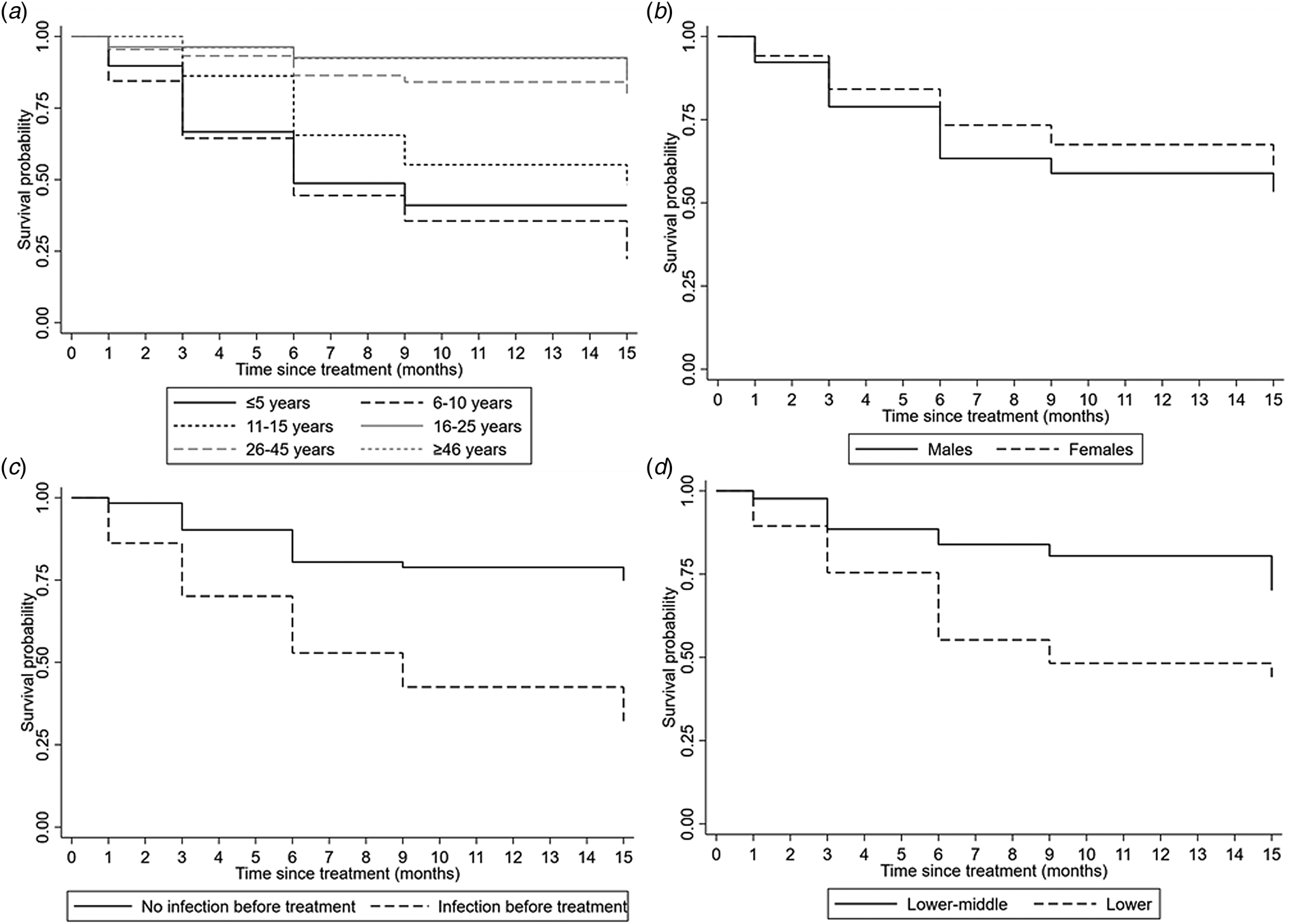
Fig. 2. Kaplan-Meier survival curves for A. lumbricoides infections over time since anthelmintic treatment according to age (a), gender (b), previous infection before treatment (c) and socio-economic status (d).
Table 3. Significant results of the Cox proportional hazards regression model for A. lumbricoides (re)infection after treatment.
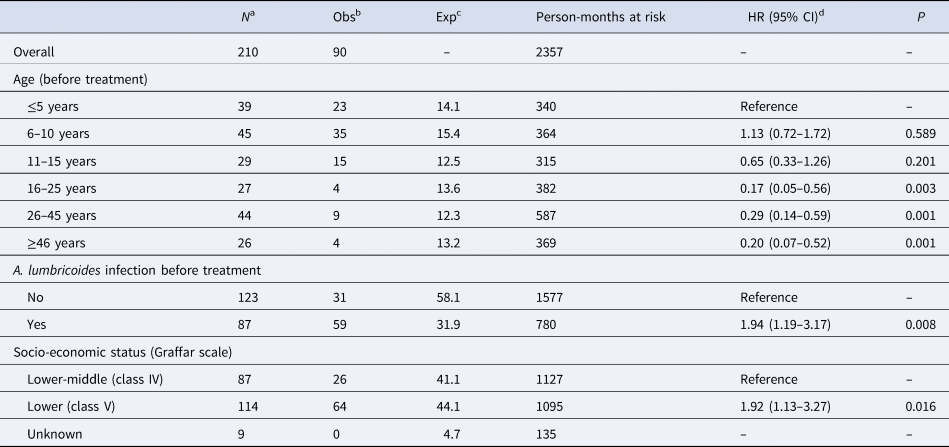
a Number of participants that received treatment with observations not beginning on or after infection.
b Observed number of A. lumbricoides infections.
c Expected number of A. lumbricoides infections.
d HR, hazard ratio, 95% CI, 95% confidence interval. Estimates are adjusted for the variables included in the table and for clustering of participants at the household level; months after treatment is the underlying time scale.
Discussion
In this study, effects of age, sex, SES and pre-treatment infection status were observed on the risk of A. lumbricoides (re)infection after treatment. Older age appeared to be a protective factor for A. lumbricoides in terms of both occurrence and intensity of infection. A reduced prevalence, but not intensity of infection, was observed in females compared to males. Increased prevalence and intensity of infection were found to be associated with lower SES. Moreover, the rate of A. lumbricoides (re)infection after treatment was higher within younger age groups, lower SES and presence of prior A. lumbricoides infection.
Age is perhaps the most common predictor of STHs in general and defines clear target groups with the highest parasitic load in an endemically infected community. Typically, the age-related intensity of infection in most settings shows a peak at 5–10 years of age for A. lumbricoides [Reference Elkins, Haswell-Elkins and Anderson27, Reference Bundy and Medley28]. Also in this study, A. lumbricoides prevalence peaked in children of 6–10 years of age, with significantly lower prevalence in older age groups. Furthermore, the intensity of infection (and hence the capacity of polluting soil with parasite eggs), was significantly higher in <10 year old children, which accounted for 60% of the total egg output in the community. These findings suggest that A. lumbricoides control programmes based on mass drug administration would be more effective when targeting children of 2–12 years of age in resource-limited settings, as suggested by the WHO Roadmap for STH control (9).
Regarding sex differences in STH prevalence, a meta-analysis has shown a significantly higher prevalence of A. lumbricoides in females [Reference Wright29]. However, in this study, we detected a significantly lower prevalence in females than males. These results may be a reflection of lower exposure to the parasites among females in the community, as males in this rural community are typically more likely to engage in leisure or agricultural activities that bring them to closer and more frequent contacts with soil. Nevertheless, sex difference was not found for parasitic loads.
SES has often been advocated as a proxy for the high prevalence and intensity of infection for STHs. Indeed, several studies have found a relation between poverty, poor housing conditions, overcrowding, lack of basic services, and means of faecal disposal, and have been associated with spatial clustering of infections in certain households [Reference Walker, Hall and Basanez19, Reference Elkins, Haswell-Elkins and Anderson27, Reference Pullan30–Reference Halpenny33]. In the current study, some of these factors were found to be significantly associated with the participant's ‘worminess’ [Reference Elkins, Haswell-Elkins and Anderson27]. The community under study has seen a decline in living conditions in the past 15 years. Indeed, the descendants of the founder population had reasonable State-built rural housing in the 1980s, but they faced severe deterioration of living conditions later on (e.g. irregular indoor water supply, and consequently no or little use of toilets) as a result of the political and socio-economic crisis affecting the country. Furthermore, the subsequent generation of community residents has had little opportunity to access proper housing and sanitisation, but rather live in self-build houses made of waste material with virtually no sanitation. It is therefore conceivable that several of such SES-related components measured by the Graffar system acted as a proxy for the risk of A. lumbricoides infection in our study.
Pyrantel is usually highly effective against A. lumbricoides at a single dose [Reference Keiser and Utzinger17], as also shown in this study. Similar to findings reported elsewhere [Reference Walker, Hall and Basanez19, Reference Jia21], the community prevalence returned to almost pre-treatment levels at six months post-treatment. The intensity of infection showed a similar pattern, returning to pre-treatment levels at nine months after treatment. These results, when compared to those obtained with albendazole or mebendazole treatment [Reference Keiser and Utzinger17, Reference Yap34], question the efficacy of annual or even biannual mass drug administrations for A. lumbricoides [Reference Pullan14]. Therefore, it may be more effective, albeit more expensive, to carry out three consecutive days treatment regimens, repeated at least twice a year for a mid-term programme of five years, and to cover also STHs other than A. lumbricoides, such as Trichuris trichiura and hookworms [Reference Steinmann18–Reference Jia21], which are other two STHs included in the WHO Roadmap for Neglected Tropical Diseases [9], thereby including enough time to develop the means of sanitation [Reference Yap34, Reference Steinmann35].
Survival analysis showed that the rate of (re)acquisition of A. lumbricoides infection after treatment was slower with increasing age. Indeed, by the end of the 15-month study period, the probability of being infection-free among children was below 50%, whereas it remained above 75% among adults. Similar age effects have been described before [Reference Jia21, Reference Bundy and Medley28], and are related to both immunity and increased exposure to A. lumbricoides among children. Yet, the strongest predictor was having had an A. lumbricoides infection before the treatment, suggesting an intrinsic proneness to A. lumbricoides infection in some individuals, the so-called ‘wormy’ persons. These findings support the importance of young age in defining the targets of mass drug administration programmes, but on the other hand, they highlight that STHs tend to cluster and recur in some people [Reference Elkins, Haswell-Elkins and Anderson27, Reference Wright29]. The reasons as to why some individuals tend to acquire the infection more frequently than others can be both epidemiological (increased exposure to the parasite, poor hygiene, etc.) and biological (e.g. increased susceptibility to infection), and these persons are the largest contributors to the environmental pollution with worm eggs [Reference Walker, Hall and Basanez19, Reference Jia21, Reference Elkins, Haswell-Elkins and Anderson27, Reference Wright29, Reference Forrester36–Reference Mehta38]. Household clustering of wormy persons has been described before [Reference Forrester15, Reference Chan, Kan and Bundy16, Reference Elkins, Haswell-Elkins and Anderson27, Reference Wright29], highlighting the potential of targeted control efforts in rural communities. However, it is also true that given the pre-patent period of A. lumbricoides, some of the infections observed one month after treatment might be persistent infections that were not cleared by the treatment rather than new infections. Regardless, their impact can be generally expected to be limited, as the large majority (86.2%) of the participants that were positive before the treatment were found to be negative one month after the treatment, meaning that ≤13.8% of them may be persistent infections contributing to the relatively low community prevalence observed at one month post-treatment. While this study cannot fully discern whether these were new or persistent infections, the analyses included the pre-treatment infection status of the participants as a variable of interest in order to take into account the effect of having had the infection previously. Other factors that might influence the pre-patent period like age were also taken into account. Moreover, although the presence of possible persistent infections needs to be considered in the interpretation of some results, it also offers the opportunity to observe the natural rebuilding of the community levels of A. lumbricoides infection in a real-world setting where inevitably an imperfect treatment could be applied. Cross-infection of humans with Ascaris suum from pigs cannot be excluded as well. Indeed, there is evidence indicating that cross-infection may occur under certain circumstances, particularly in industrialised countries where A. lumbricoides occurs very rarely, but pig infection with A. suum is common. Humans have therefore been found to be infected and molecular markers have shown that the worms responsible for such infection are genetically close to A. suum [Reference Peng and Criscione39]. Pigs bred domestically, with basically no biosecurity measures in place, are common in the sampled community. Consequently, it cannot be excluded that cross-infection of humans with A. suum occurred.
A final consideration pertains to the time elapsed between data collection and analysis (11 years). However, because of the protracted socioeconomic and political crisis in the country, it is unlikely that the dynamics of STHs have changed in the study area, as no improvements of the country and local living conditions have occurred ever since [Reference Grillet40–43]. Moreover, our observations might be generalisable to similar situations in other resource-limited settings worldwide and provide historical data for comparison with other studies as well.
In conclusion, younger age, lower SES and pre-treatment infection status are significant predictors of A. lumbricoides (re)infection after treatment, the burden of which seems to regain its community baseline levels after six months. Our findings seem to suggest that mass drug administration protocols would benefit from considering these factors in selective treatment strategies and possibly more than just annual or biannual treatments in the target population.
Acknowledgements
The authors would like to thank the personnel involved in the field and laboratory work: laboratory technicians Esmirna Colmenares, Milagro Armas, María Isabel Simmons and Gleisa Medina, from the Department of Parasitology, Faculty of Health Sciences, Universidad de Carabobo, Valencia, Venezuela.
Financial support
Financial support was received from the project LOCTI-Universidad de Carabobo N° 1.235 under the sponsorship of the enterprise Nascar Autopartes.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
The study adhered to local ethical criteria and was approved by the Ethical Committee of the Carabobo State Health Authority (INSALUD), Venezuela, and by the Ethical Committee of VU University, Amsterdam, the Netherlands.
Data availability statement
All data relevant to the study are included in the article in an aggregated and anonymised format. For legal reasons, the disaggregated dataset is available in an anonymised format from the first author on reasonable request.








