Introduction
In December 2019, Wuhan, China had reported a cluster of unexplained cases of viral pneumonia. This disease was soon named as coronavirus disease 2019 (COVID-19), and determined to be caused by a novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. In the past two months, COVID-19 has spread across the globe. According to data released by the World Health Organization (WHO), as of 10:00 on 4 April, SARS-CoV-2 had infected 207 countries, areas or territories with a total of 1 051 697 confirmed cases and 56 986 deaths worldwide [2]. The confirmed cases in America, Italy and Spain have surpassed 100 000 and the cases continue to increase rapidly in across the world [3]. It has become a serious threat to global health and a significant challenge to health care systems worldwide.
While the disease is mild or even asymptomatic in most patients, and usually self-resolves without the need for hospitalisation, there was still a certain proportion of severe cases. The treatment of severe cases was difficult and the fatality rate was high. As of 16 February, China's COVID-19 epidemic report data showed that 19.6% of patients were severe cases [Reference Yu4], and the fatality rate of these cases was 49% [5]. Furthermore, a study included 52 severe case patients showed that the fatality rate was as high as 61.5% [Reference Yang6]. Therefore, it is critical to understand and identify the risk factors for the progression of COVID-19 patients in order to help in early identification of severe cases and improving the prognosis of patients.
Two recent systematic study reviews [Reference Lippi7,Reference Lippi8] of COVID-19 patients indicated increased procalcitonin values that were associated with a nearly five-fold higher risk of severe infection and low platelet count was associated with increased risk of severe disease and mortality in patients with COVID-19. However, both reviews meta-analysed small samples pooled from few studies and the indicators were not comprehensive. Recently, many large-scale clinical studies have been published [Reference Deng9–Reference Zhang12], but the results across these studies were not entirely consistent. In order to gain a clearer picture of the risk factors of severe COVID-19, we meta-analysed the relevant literature. The results may provide a basis for detecting or even predicting disease progression quickly enough to improve prognosis.
Materials and methods
Search strategy
This meta-analysis was carried out according to preferred reporting items for Meta-Analyses of Observational Studies in Epidemiology (MOOSE) statement [Reference Stroup13]. PubMed, Web of Science, Scopus, EMbase, CNKI, WanFang Data, Chinese Biomedical Literature Database and VIP databases were electronically searched to collect clinical studies of severe or critically ill COVID-19 patients from 1 January 2020 to 3 April 2020. We also manually searched the lists of included studies to identify additional potentially eligible studies. If there were two or more studies that described the same population, only the study with the largest sample size was chosen. There was no language restriction placed in the literature search, but only literature published online were included. The following keywords were used, both separately and in combination, as part of the search strategy in each database: ‘Coronavirus’, ‘2019-nCoV’, ‘COVID-19’, ‘SARS-CoV-2’, ‘severe’, ‘critical’, ‘icu care’, ‘mechanical ventilation’, ‘intensive care unit’, ‘mortality’, ‘fatal’, ‘death’, ‘survivors’ or ‘critically ill’.
Study Eligibility
Studies were included in the meta-analysis if they had cohort, case−control or case-series designs; if they contained patients with mild and severe disease, or survivor and death groups; the laboratory outcomes of the COVID-19 patients included in our study were the findings when they were admitted to the hospital or first visited the hospital. At the end of the follow-up, the patients were divided into mild and severe groups. We considered disease to be ‘mild’ in those patients described in the studies as having mild or moderate disease, or ‘severe’ in those patients described as having severe disease, as being admitted to the intensive care unit or as requiring mechanical ventilation. Only studies of more than 30 patients were included.
Data extraction and quality assessment
Three reviewers independently selected literature and extracted data to an Excel database. Any disagreement was resolved by another reviewer. The titles and abstracts were first screened to identify the eligible articles, followed by a full-text review to obtain detailed information. When required, the authors were contacted directly to obtain further information and clarifications regarding their study. Data extraction included: The first author's surname and the date of publication of the article, study design, sample size, age, outcome measurement data such as laboratory findings reported in the identified papers, relevant elements of bias risk assessment.
The quality of included studies was independently evaluated by the three reviewers based on the Newcastle-Ottawa Scale (NOS) [Reference Stang14] guidelines. Any disagreement was resolved by another reviewer. Studies with a score greater than 6 were considered to be of high quality (total score = 9).
Statistical analyses
Data from studies reporting continuous data as ranges or as median and interquartile ranges were converted to mean ± s.d. [Reference Hozo15]. The weighted mean differences (WMDs) in continuous variables between patient groups were calculated, together with the associated 95% confidence intervals (CIs). All meta-analyses were performed using STATA 12 (StataCorp, TX, USA). Since all studies were gathered from the published literature and the sample size of included studies varies greatly, so a random-effects model was used. Funnel plot together with Egger's regression asymmetry test and Begg's test were used to evaluate publication bias. A two-tailed P < 0.05 was regarded as statistically significant.
Results
Literature screening and assessment
A total of 4122 records were identified from the databases. In addition, 204 records were identified from the Chinese Medical Journal Network. After a detailed assessment, 40 studies [Reference Yang6, Reference Deng9–Reference Zhang12, Reference Zhou16–Reference Bin50] involving 5872 COVID-19 patients were included in the meta-analysis (Fig. 1).

Fig. 1. Flowchart depicting literature screening process.
Characteristics of included studies
All studies included in the meta-analysis were conducted in China examined Chinese patients distributed across 31 provinces and published between 8 February 2020 and 2 April 2020. A large proportion of these studies (n = 37) were based on data collected from a single centre. Follow-up data were reported for most patients. All studies received quality scores of 6–9, indicating high quality (Table 1).
Table 1. Basic characteristics of included studies of COVID-19 patients in China

a All studies were retrospective cohort studies.
b Reported as range, mean ± SD, or median (interquartile range). NR, not reported.
c Score based on the Newcastle−Ottawa scale guidelines [Reference Stang14].
Meta-analysis
Age distribution
A total of 29 studies involving 3411 COVID-19 patients were included. Although the heterogeneity was high across enrolled studies, the result showed that compared with non-severe group, the age of severe group was higher (WMD = 10.69, 95%CI 7.83–13.54) (Fig. 2).
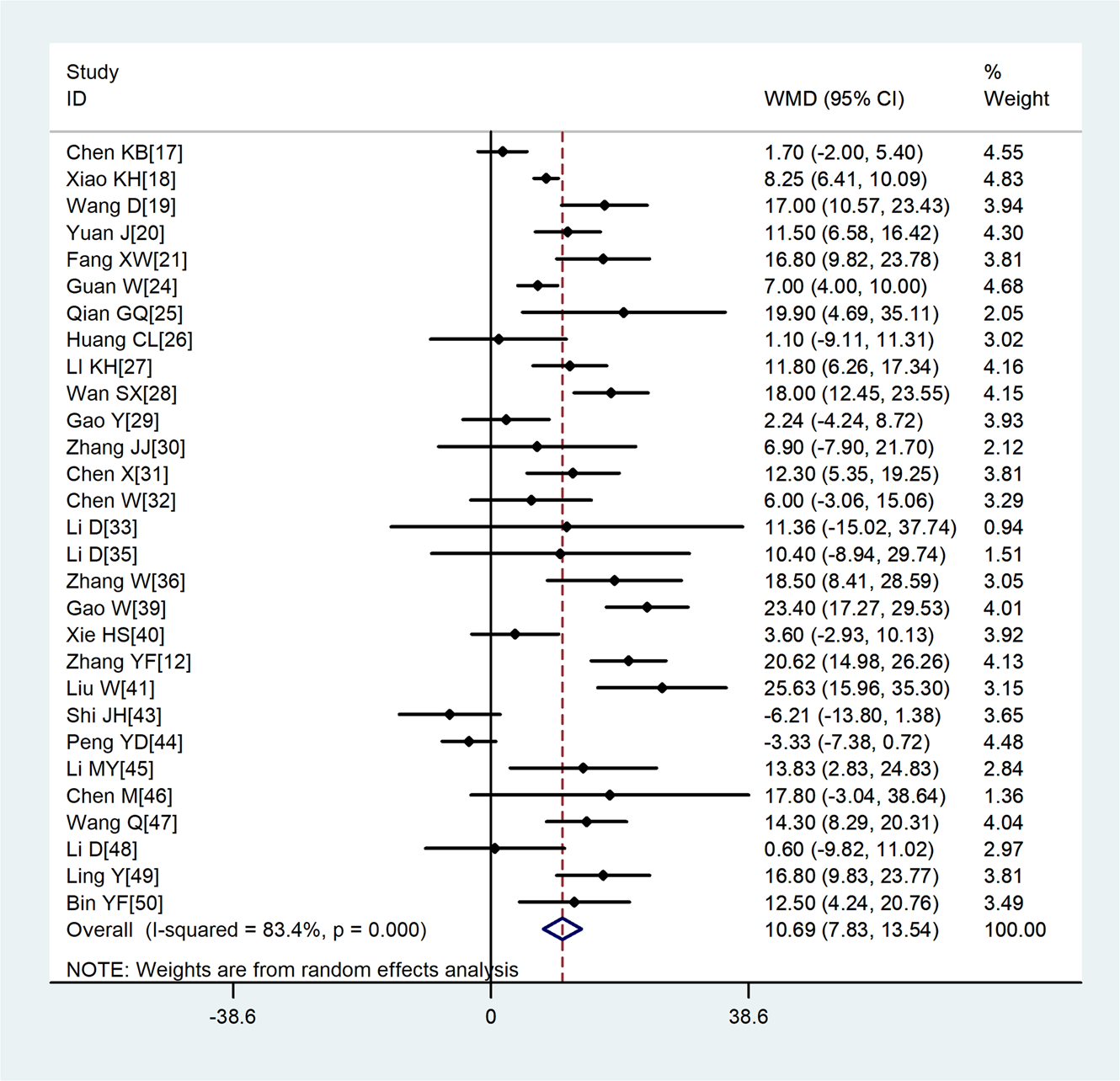
Fig. 2. Meta-analysis of the difference in the average age between COVID-19 patients with mild or severe disease. WMD, weighted mean difference.
Laboratory parameters
Compared with non-severe group, the lymphocyte count (WMD = −0.35, 95%CI −0.41 to −0.30) and the platelet count (WMD = −18.63, 95%CI −30.86 to −6.40) were found to be lower, while C-reactive protein (CRP; WMD = 42.7, 95%CI 31.12–54.28) and lactate dehydrogenase (LDH; WMD = 137.4, 95%CI 105.5–169.3) were significantly higher in the severe group (Figs 3–6). Patients in the severe group also displayed elevated levels of white blood cell count (WBC; WMD = 0.93, 95%CI 0.51–1.36), procalcitonin (PCT; WMD = 0.07, 95%CI 0.05–0.10), D-dimer (WMD = 0.38, 95%CI 0.24–0.52), alanine aminotransferase (ALT; WMD = 5.12, 95%CI 0.82–9.42), aspartate aminotransferase (AST; WMD = 8.51, 95%CI 5.01–12.01) and creatinine (Cr; WMD = 4.57, 95%CI 0.64–8.50) compared to those in the non-severe group (Table 2).
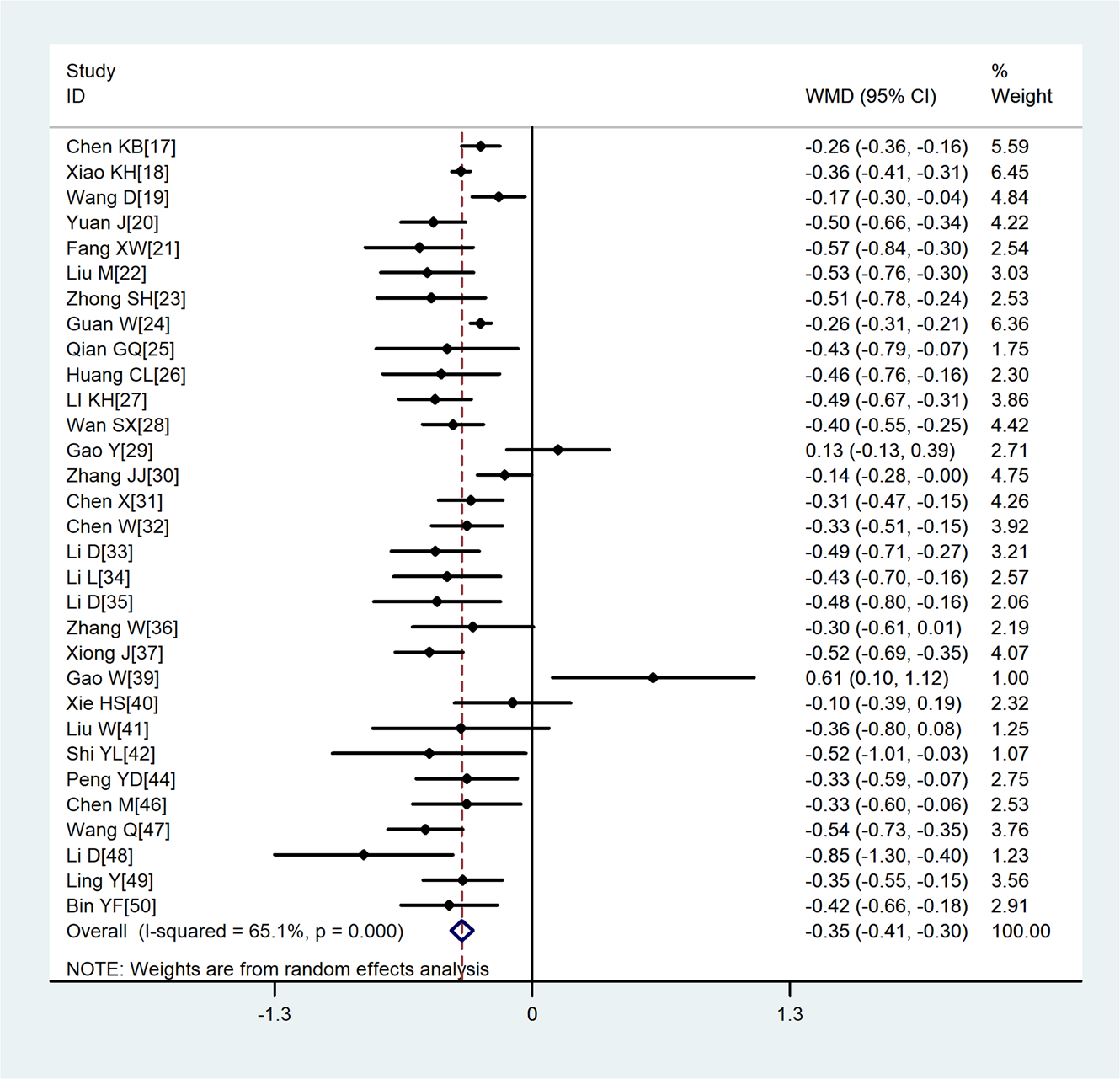
Fig. 3. Meta-analysis of the difference in the lymphocyte count between COVID-19 patients with mild or severe disease. WMD, weighted mean difference.
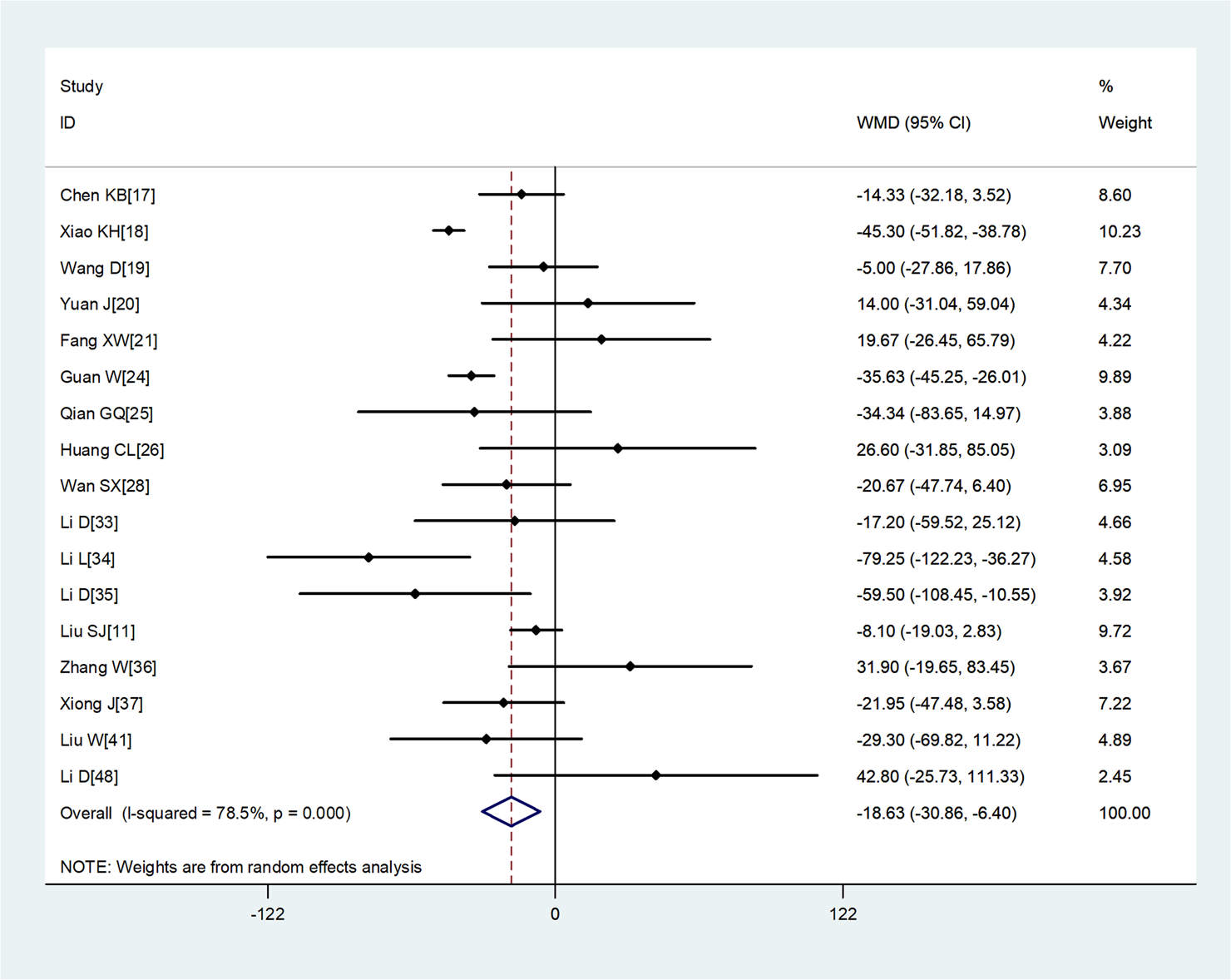
Fig. 4. Meta-analysis of the difference in the platelet count between COVID-19 patients with mild or severe disease. WMD, weighted mean difference.
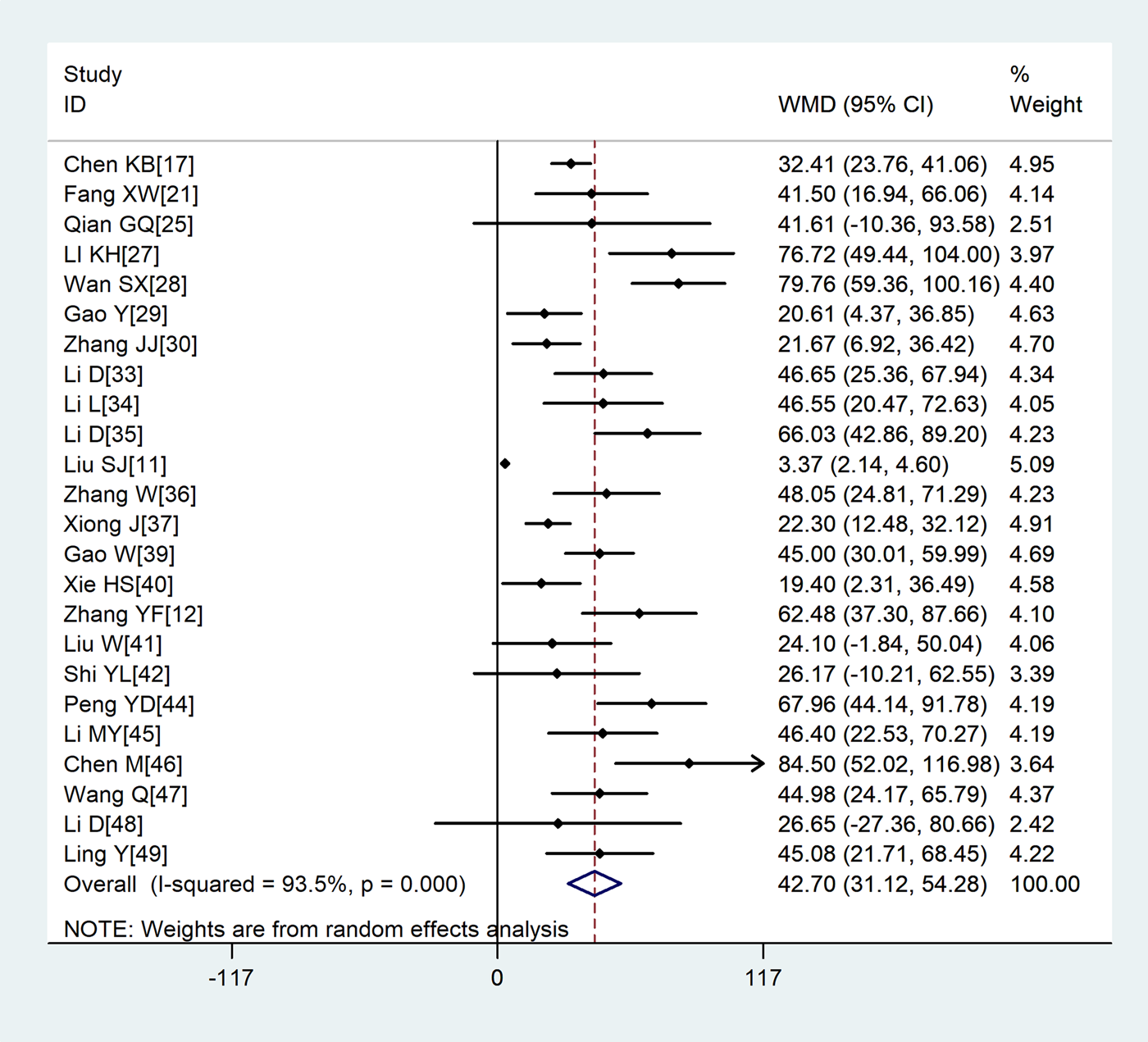
Fig. 5. Meta-analysis of the difference in the C-reactive protein between COVID-19 patients with mild or severe disease. WMD, weighted mean difference.
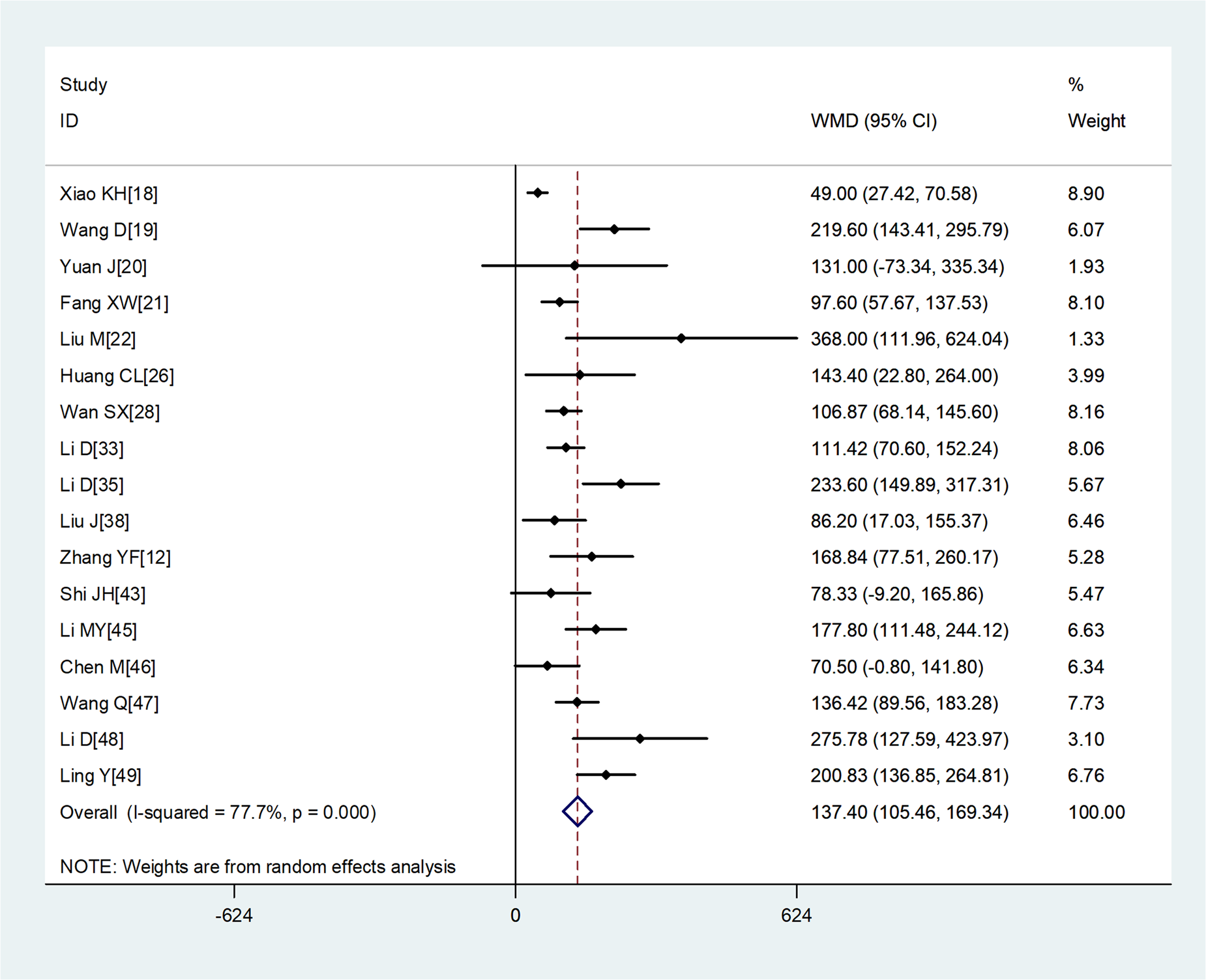
Fig. 6. Meta-analysis of the difference in the lactate dehydrogenase between COVID-19 patients with mild or severe disease. WMD, weighted mean difference.
Table 2. Meta analysis of different laboratory parameters in COVID-19 patients
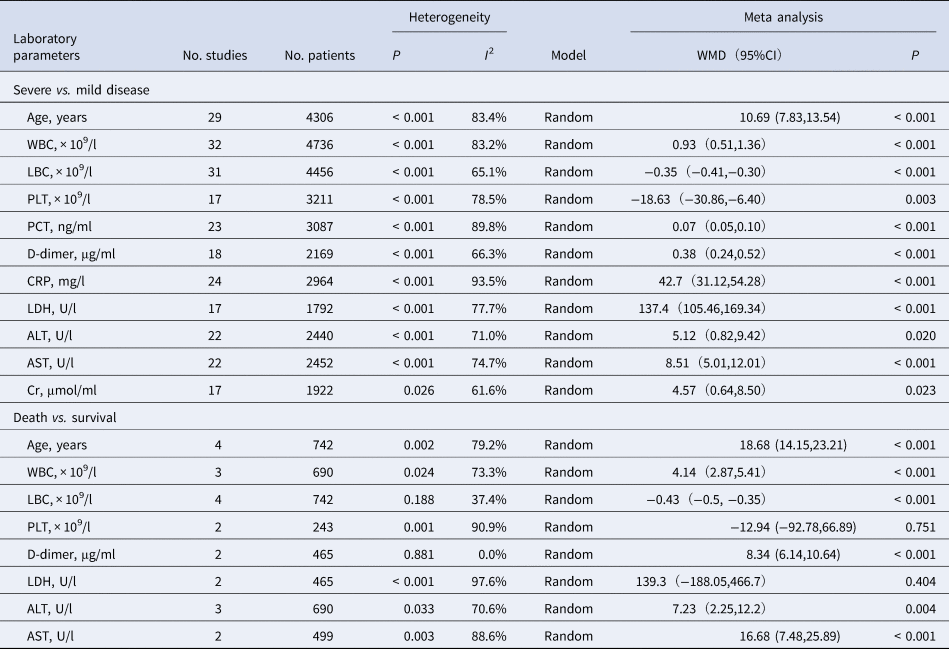
CI, confidence interval; WMD, weighted mean difference.
Four studies [Reference Yang6, Reference Deng9, Reference Chen10, Reference Zhou16] whose primary outcome was death were also analysed. The results showed that on admission, patients who died showed significantly higher WBC, D-dimer, ALT, AST and Cr but similar platelet count and LDH as patients who survived (Table 2).
Sensitivity analysis
To determine sensitivity, we removed each study one by one and the pooled results did not change substantially, indicating the reliability and stability of our meta-analysis (e.g. Figure 7).

Fig. 7. Sensitivity analysis of the lymphocyte count between COVID-19 patients with or without severe disease.
Publication bias
The P values derived using the Egger's and the Begg's test for all outcomes showed no obvious publication bias (Table 3). A funnel plot based on the outcome of lymphocyte count showed the P-values of Egger's and Begg's test were 0.315 and 0.919, respectively, indicating that the publication bias did not exist (Fig. 8).
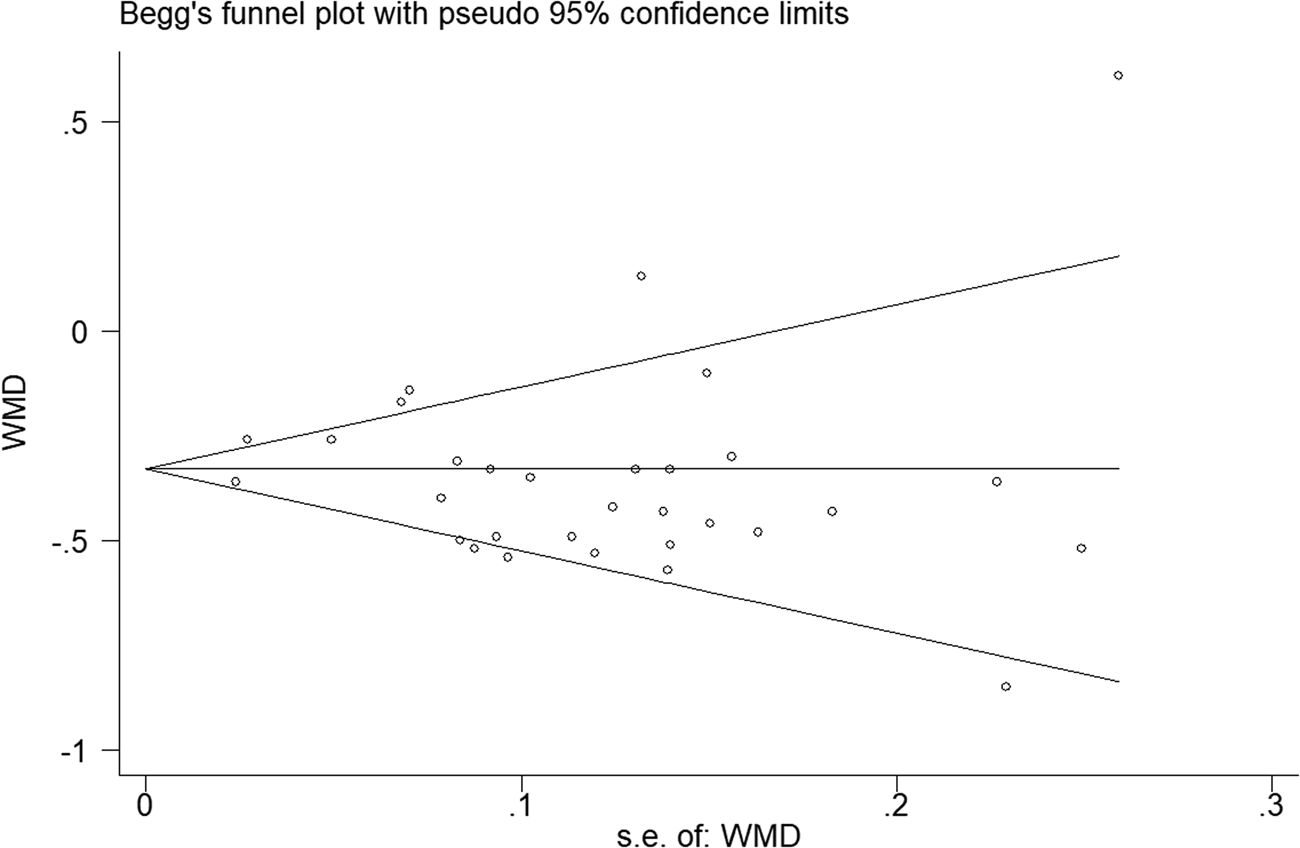
Fig. 8. Funnel plot regarding the outcome of lymphocyte count.
Table 3. Evaluation of publication bias using the Egger's and the Begg's test

Discussion
In this study, we meta-analysed the relevant literature from 1 January 2020. Our analysis of 40 studies [Reference Deng9–Reference Zhang12, Reference Hozo15–Reference Bin50] involving 5872 COVID-19 patients suggests that lymphocyte and platelet count were found to be lower in those with severe disease than in those with mild disease, and significantly lower in those who die during follow-up than in those who survive. One plausible explanation is severely impaired immune function in severe cases, accompanied by lymphocyte necrosis and apoptosis, resulting in decreased lymphocytes in peripheral blood. According to the study by Zarychanski et al. [Reference Zarychanski51], thrombocytopenia was commonplace in severe or critically ill patients, and usually suggests serious organ malfunction and may evolve towards disseminated intravascular coagulation (DIC).
We also found that LDH, ALT, AST and Cr were higher in severe or death group, which suggested that the heart, liver, kidney and other important organ functions were more severely damaged in severe patients. Studies have shown that elevated levels of LDH was a risk factor for mild patients progressing to become critically ill patients [Reference Phan52] and the incidence of myocardial injury was greater in severe patients [Reference Li45]. A recent meta-analysis included 341 COVID-19 patients, and the results showed that the values of cTnI were found to be significantly increased in COVID-19 patients with severe disease than in those without (SMD = 25.6, 95% CI 6.8–44.5) [Reference Lippi53]. According to Xie et al. [Reference Xie40], liver injury was common in hospitalised COVID-19 patients, and it may be related to systemic inflammation. Therefore, intense monitoring and evaluation of liver function in COVID-19 patients should be considered. In addition, PCT and CRP were higher in the severe cases of this study. Since the production and release into the circulation of PCT from extrathyroidal sources is enormously amplified during bacterial infections [Reference Lippi7], suggesting that severe cases were more likely to have a bacterial infection, so serial PCT measurement may play a role for predicting evolution towards a more severe form of the disease.
According to the study by Mahase [Reference Mahase54], the overall fatality rate in COVID-19 patients has been estimated at 0.66%, rising sharply to 7.8% in people aged over 80 and declining to 0.0016% in children aged 9 and under. In Italy, the case-fatality rate even reached 20.2% in people aged over 80 [Reference Onder55]. In our study, severe patients were older compared to non-severe patients. These results suggest that older age is associated with an increased risk of death. The underlying reasons may be that older age had a more significant number of comorbid conditions such as hypertension and diabetes mellitus, most of the chronic diseases share several standard features with infectious disorders, such as the proinflammatory state, and the attenuation of the innate immune response. Therefore, older age and comorbidities could be risk factors for severe patients.
Although our meta-analysis rigorously analysed data from a large sample of COVID-19 patients, our results are limited by the heterogeneity observed across studies. For example, given that most of the studies included in our meta-analysis were single-centre, retrospective studies, it was difficult for us to control for the effects of several confounding factors, including the disease course and severity, the participants' inclusion criteria as well as the studies design. Additionally, the studies included in our meta-analysis were from China, not those infected in other countries, so geographical and ethnic differences were not excluded whether the conclusion was consistent in other countries needs to be further investigated.
Conclusion
In summary, current evidence showed that, older age, low platelet count, lymphopenia, elevated levels of LDH, ALT, AST, PCT, Cr and D-dimer were associated with severity of COVID-19. And thus could be used as early identification or even prediction of worsening illness. Due to the limited quality and quantity of the included studies, more high-quality prospective studies are required to verify the above conclusions.
Acknowledgements
This study was supported by grants from the Emergency Science and Technology Brainstorm Project for the Prevention and Control of COVID-19, which is part of the Guangxi Key Research and Development Plan (Guike AB20058002).
Conflict of interest
The authors have declared that no competing interests exist.
Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.














