INTRODUCTION
Human salmonellosis is a notifiable disease in Ontario and Canada and is the second most frequently reported enteric bacterial illness after campylobacteriosis, with ∼5000 cases reported annually in Canada. During 2003–2009, the national annual incidence rate of Salmonella serovar Enteritidis (SE) increased from 2·16 to 5·79/100 000 person-years [Reference Nesbitt1]. In 2003, SE represented 12·7% of all Salmonella cases, increasing to 32·1% in 2009. Further, due to underreporting, for every Salmonella case reported, it is estimated that 13–37 cases occur annually in the Canadian population [Reference Thomas2]. Therefore, salmonellosis, and SE specifically, represents a significant health burden to Canadians.
Ontario is Canada's largest province, consisting of an estimated 13·4 million residents in 2011 [3]. Similar to national trends, the number of SE cases has increased steadily in Ontario over the last decade. Following a large outbreak of SE in 2005, the average annual number of SE cases remained elevated at 710 for 2006–2009 compared to the pre-outbreak annual average of 502 from 2000 to 2004 [4–10]. A marked increase in late 2009/early 2010 prompted a more detailed investigation of SE cases in Ontario. The case total for 2010 was 1035 [11]. Prior to undertaking a case-control study, questionnaires were administered to case-patients with laboratory-confirmed illness in 2010 to generate hypotheses about the risk factors for illness. During this period, phage types (PTs) 13, 8, and 13a were the most prevalent experienced in Ontario. It was hypothesized that these phage types were associated with domestic rather than travel-related infections.
Historically, SE has been commonly linked to eggs and more recently chicken meat [Reference Nesbitt1, Reference Altekruse12–Reference Berrang16]. The primary purpose of the investigation was to identify the cause(s) of the increase in the number of SE cases in Ontario. Travel-related illness accounted for a large proportion of the cases and contributed in part to the increase; these results are described in another publication [Reference Tighe17]. In general, the purpose of this study was to identify risk factors for acquiring illness due to SE within Ontario (i.e. domestically acquired). Following our hypothesis-generation analysis, it was shown that poultry meat and processed chicken (e.g. frozen chicken strips, chicken nuggets, chicken burgers) were hypothesized to be risk factors for human illness due to SE. The purpose of this study was to determine whether any poultry meat consumption and processed chicken consumption, identified in the hypothesis-generating phase of the study, were risk factors for acquiring illness due to SE within Ontario through the use of a prospective case-control study design.
METHODS
Laboratory testing
The Public Health Ontario laboratories (PHOL) consist of one central (i.e. Toronto) and 10 regional laboratories. These laboratories serve as the reference centre for enteric bacteria in Ontario. All isolates of Salmonella are forwarded to the Toronto Public Health Laboratory (TPHL) for culture confirmation using biochemical assays and serotyping based on the Kauffmann–White scheme [Reference Grimont and Weill18]. As per routine practice, all isolates identified as SE are forwarded to the National Microbiology Laboratory in Winnipeg for phage typing using methods described by Ward and colleagues [Reference Ward, de Sa and Rowe19].
The laboratory and phage-typing results are compiled daily at the TPHL and shared with the investigators in a line list format.
Study design
In preparation for the case-control study, hypothesis generation was conducted through the administration of questionnaires to case-patients with laboratory-confirmed Salmonella from 12 July to 10 December 2010. The purpose of the interviews was to generate hypotheses about the risk factors for acquiring SE in Ontario, to refine the questionnaire for use in the case-control study, and to assist with calculating the sample size required for adequate statistical power in the case-control study. A prospective case-control study was then conducted from 20 January to 12 August 2011.
Cases
All incident cases with culture-confirmed SE from faecal samples received at the TPHL from 20 January to 12 August 2011 were considered for the study. The majority of case-patients were interviewed by one staff member at Public Health Ontario. The remaining case-patients were interviewed by two other staff members. Case-patients were excluded prior to being interviewed who: resided outside of Ontario or had SE isolated from a clinical specimen other than stool. Case-patients were lost to follow-up that did not have a telephone number available, could not be reached following five attempts, or died. Refusals were defined as those case-patients who declined to be interviewed. Case-patients were excluded after being contacted that resided on a First Nations reserve, were asymptomatic, could not recall their symptom onset date, had testing performed more than 2 months following symptom onset, were a secondary case (e.g. they lived with another person who had similar symptoms in the week prior to their symptom onset), were part of a recognized cluster or outbreak (not including the index case), had travelled outside Canada and the USA within the 3 days prior to symptom onset, or could not speak English. Further, only the first specimen was included for case-patients that had repeated specimens purposely performed.
Controls
Randomly selected population-based controls were frequency matched on age (<20 years or ⩾20 years) and exposure period (date of interview of controls was within 2 weeks of case symptom onset). Interviewing of controls was performed by an external service provider, the Institute for Social Research at York University, Toronto, Ontario. A three-stage probability selection process to select controls was used. First, a list of Ontario telephone numbers was constructed using telephone books and other commercially available lists of telephone numbers. Second, a random sample of telephone numbers was chosen from the list and third, eligibility of the household residents at the chosen phone numbers was determined and respondents were randomly selected. A minimum of 16 controls per month were interviewed. Controls were excluded who: could not speak English, had experienced diarrhoea (⩾2 loose stools in a 24-h period) in the preceding 3 days, had been diagnosed with Salmonella in the past 30 days, had lived with someone who had laboratory-confirmed Salmonella in the previous 30 days, or had travelled outside Canada and the USA within 7 days of the interview. The 7-day period was used rather than the 3-day period used for cases to ensure that any possible travel-associated controls were eliminated. Controls were lost to follow-up if they could not be reached following at least 20 attempts. Refusals were defined as those who declined to be interviewed.
Questionnaires and ethical approval
Telephone interviews were conducted using a standardized questionnaire that collected information on demographics, travel history, clinical symptoms (cases only), contact with animals, food exposures and food hygiene practices. Food exposures included eggs (cooked vs. undercooked or raw), poultry meat (defined as chicken, duck, geese, pheasants or turkey), processed chicken products (chicken strips, nuggets, burgers), raw milk, cheese, peanut butter, and selected raw fruits and vegetables. Cases were asked about food exposures and animal contact during the 3 days prior to their symptom onset and controls were asked about food exposures and animal contact during the 3 days prior to the interview date.
Parents or guardians responded on behalf of children aged <16 years. All respondents gave informed verbal consent prior to beginning the interview. Ethical approval was not required to interview cases because this was a public health investigation under the Ontario Health Protection and Promotion Act [20]. Ethical approval for interviewing controls was obtained from York University's Research Ethics Board (Toronto, Ontario).
Statistical analysis
We entered the case questionnaire data in EpiData version 3.1 (EpiData Entry, J. M. Lauritsen & M. Bruus). Ten percent of all case records were checked for accuracy of entry (error rate = 27/4120 or 0·7%) and changes were made to correct errors based on the paper questionnaires. In addition, range checks and tabulation of variables were performed to identify and correct any additional errors. Control data were entered as it was collected through computer-assisted telephone interviewing; data cleaning was performed to ensure skip patterns were correctly followed. Data were analysed using Stata version 10.1 (StataCorp, USA).
Age was categorized into four groups based on the age distribution of cases from the hypothesis-generating study (0–9, 10–19, 20–49, ⩾50 years), while exposure period was divided into tertiles based on the distribution of cases and controls over the study period (1 January–3 March, 4 March–14 May, 15 May–26 August). Rural status was derived from Statistics Canada 2007 peer groups reported in Ontario's Initial Report on Public Health with slight modification: peer groups A (urban/rural mix) and C (sparsely populated urban/rural mix) were collapsed into a ‘mix’ category, peer groups B (urban centre) and G (metro centre) were collapsed into an ‘urban’ category and peer groups E (mainly rural) and H (rural northern) were collapsed into a ‘rural’ category [21].
We first explored associations between the study exposure variables and endemic SE infection (all phage types combined) through univariable analyses using Pearson's χ 2 test and/or Fisher's exact test. Cases with missing data or who did not know whether they had consumed a specific food exposure were excluded.
To examine exposures of interest from the univariable analysis, two multivariable unconditional logistic regression models were built to examine our hypotheses of poultry meat and processed chicken consumption using a forward fitting approach. Age group and exposure period were added to the model as a priori confounders, along with other exposure variables that were either associated with the outcome of interest at the P ⩽0·20 level or that had an odds ratio >2·5 in the univariable analysis. Variables were added to the model in order of their effect size and retained in the model if they modified the effect size of the association between the exposure of interest and the outcome by >10% or if the introduction of the variable had substantial effects on the confidence interval. After variable selection was performed, interaction between the exposure of interest and age was tested through the addition of interaction terms to the model; statistical significance was assessed using likelihood ratio tests. Odds ratios were used as measures of effect size (or association) in this study.
We also explored associations between exposures and specific phage typres (8, 13, 13a); however, we did not find any unique relationships that were not already demonstrated in the overall analysis with the exception of PT13. The unique associations identified for PT13 are described in the Results section.
Population attributable fractions were calculated for exposures in the final multivariable models using the following formula: pd((RR – 1)/RR), which uses adjusted relative risks (RR), where pd = proportion of cases exposed to the risk factor [Reference Rockhill, Newman and Weinberg22]. In this study, the odds ratio was used to approximate relative risk. Confidence intervals (95%) were calculated in Stata using the ‘aflogit, cc’ command, based on methods described by Greenland & Drescher [Reference Greenland and Drescher23].
RESULTS
Case recruitment
A flow chart of case recruitment is shown in Figure 1. A total of 630 laboratory-confirmed cases of SE were identified by the TPHL between 20 January and 12 August 2011. Thirty-six cases were excluded leaving 594 cases eligible to be interviewed. A total of 87 cases were lost to follow-up and refusals. Thus, the response rate was 85·4% (507/594). Lost to follow-up and refused cases were compared to interviewed and excluded cases to assess potential biases in our data. No significant differences were detected in terms of age group (P = 0·33), sex (P = 0·17), month in which the specimen was received at the TPHL (P = 0·26) or rural status (P = 0·63). After interviewing the 507 available case-patients, a further 308 cases were excluded according to our exclusion criteria, leaving 199 SE cases included in our analysis. Of these cases, 73 (36·7%) were PT8, 50 (25·1%) PT13a, 20 (10·1%) PT13, and 56 (28·1%) other phage types.
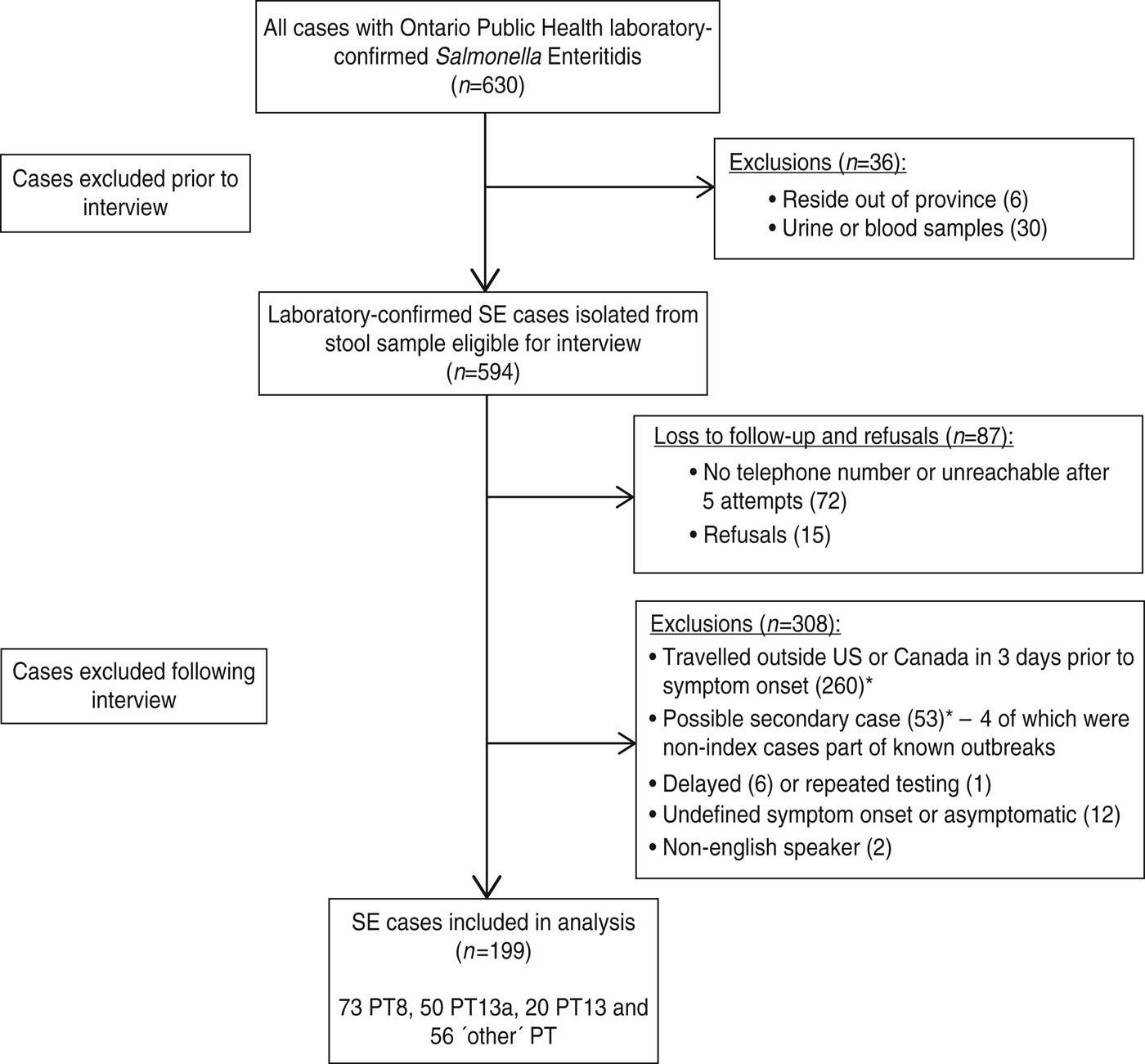
Fig. 1. Flow chart outlining Salmonella Enteritidis case recruitment. * Indicates that 26 cases were identified as both having travelled outside North America as well as being considered a potential secondary case.
Clinical characteristics of cases
Symptom information was missing for one case-patient. Of the remaining 198 case-patients, 195 (98·5%) reported experiencing diarrhoea, 179 (90·4%) abdominal cramps, 149 (75·3%) fever, 99 (50·0%) nausea, and 82 (41·4%) vomiting; additionally, 122 (61·6%) case-patients reported experiencing other symptoms than the ones listed here. Symptom onsets ranged between 2 January and 1 August 2011. For the 130 (65·6%) case-patients whose symptoms had resolved by the time of interview, the median symptom duration was 8 days. Twenty-eight (14·0%) case-patients reported being hospitalized for their illness (median duration 3·5 days, range 1–12 days).
Demographic characteristics
The demographic characteristics of case-patients and controls are shown in Table 1. Case-patients had a median age of 26 years compared to 21 years for controls; this difference was not statistically significant (P = 0·262). Similarly, no differences in sex were observed (P = 0·492). There were higher proportions of case-patients in the more urban areas of the province (P = 0·035).
Table 1. Descriptive comparison of Salmonella Enteritidis cases and controls*
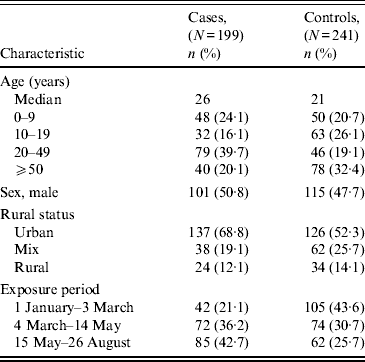
* Percentages may not total 100 because of missing data.
Risk factor findings
Table 2 presents results from the univariable analysis. Consuming any poultry meat more than doubled the odds of infection compared to not consuming it [unadjusted odds ratio (OR) 2·03, 95% confidence interval (CI) 1·26–3·28]. Additionally, consuming poultry outside the home was a risk factor for infection (OR 2·09, 95% CI 1·39–3·14).
Table 2. Proportion of cases and controls reporting various exposures in the 3 days either before illness onset or interview, along with unadjusted odds ratios
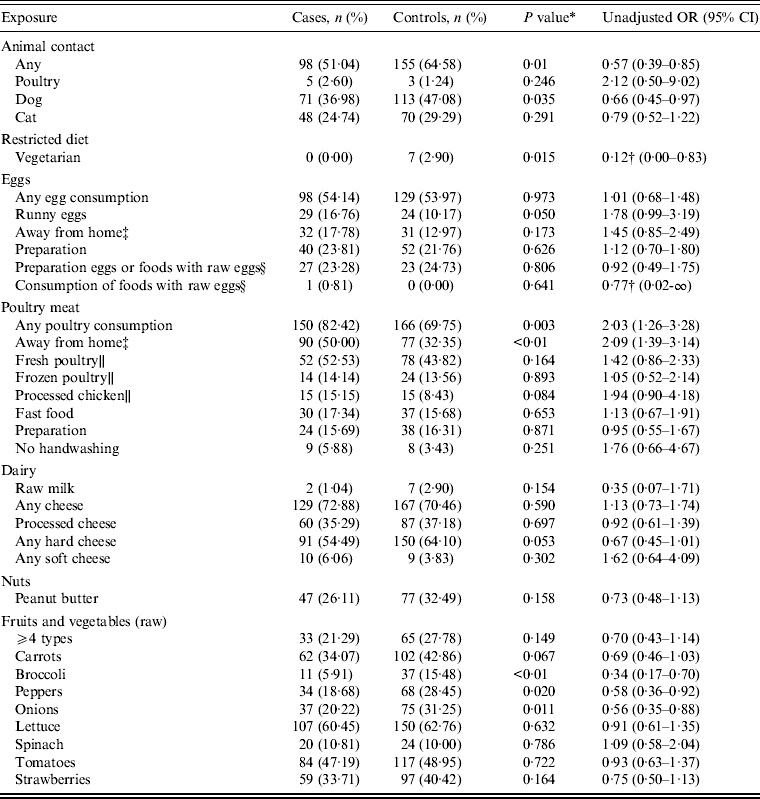
OR, Odds ratio; CI, confidence interval.
* P value from χ 2 test (a Fisher's exact test was employed where the expected cell counts were <5).
† Estimated using exact logistic regression; represents the median unbiased estimate.
‡ Only asked of respondents who reported consuming either eggs or poultry.
§ Only asked of respondents from 31 March onwards (N = 233).
∥ Respondents who reported only consuming poultry away from home are excluded from the denominator (N = 118).
To further investigate whether the odds of infection varied by type of poultry meat consumed, additional questions were asked to all respondents with the exception of those who only reported consuming poultry outside the home (n = 118). Consuming processed chicken was reported by 15·15% of cases and 8·43% of controls (OR 1·94, 95% CI 0·90–4·18). Although this unadjusted odds ratio was not significant for all phage types combined, we found that consuming processed chicken significantly increased the odds of infection for PT13 (OR 5·43, 95% 1·42–20·81). While the consumption of poultry that was purchased fresh (OR 1·42, 95% CI 0·86–2·33) presented a higher odds than frozen poultry (OR 1·05, 95% CI 0·52–2·14), neither were statistically significant risk factors.
Additional questions on egg preparation, consumption of food items or drinks that contained raw or uncooked eggs, and behaviours related to hand washing following handling of raw eggs were asked for a subset of respondents (e.g. all respondents with an exposure time beginning 31 March 2012 onwards, n = 233). In this subset, we found that not washing hands following handling of raw eggs almost tripled the odds of infection relative to those who reported washing their hands (OR 2·82, 95% CI 1·48–5·37). Preparing eggs or food items containing raw eggs was not associated with infection (OR 0·92, 95% CI 0·49–1·75). Only one case reported consuming food or drink that contained raw eggs; no controls reported this exposure.
Exposures that had statistically significant unadjusted odds ratios that were protective included: any animal contact (OR 0·57, 95% CI 0·39–0·85), contact with a dog (OR 0·66, 95% CI 0·45–0·97), broccoli (OR 0·34, 95% CI 0·17–0·70), peppers (OR 0·58, 95% CI 0·36–0·92), and onions (OR 0·56, 95% CI 0·35–0·88). For PT13 specifically, we found statistically significant protective odds ratios for the following exposures: contact with a cat (OR 0·13, 95% CI 0·02–0·99) and hard cheese consumption (OR 0·34, 95% CI 0·12–0·97). None of the cases in this study were vegetarian.
Overall, any poultry meat consumption was independently associated with SE infection; this association persisted after adjustment for age, exposure time period and egg consumption [Table 3, adjusted odds ratio (aOR) 2·24, 95% CI 1·31–3·83]. Similarly, in the subset of individuals who consumed any poultry at home, processed chicken consumption was independently associated with SE infection after controlling for age group, exposure time period, animal contact, consumption of hard cheese and raw carrots (Table 3, aOR 3·32, 95% CI 1·26–8·76). There was no evidence of interaction with age.
Table 3. Final multivariable models for main hypothesized exposures

OR, Odds ratio; CI, confidence interval.
* Adjusted for age group, exposure time period and egg consumption.
† Adjusted for age group, exposure time period, animal contact, hard cheese consumption and raw carrot consumption.
The population attributable fraction for any poultry meat consumption was 46% (range 18–64%), while the population attributable fraction for processed chicken consumption was 10% (0–19%).
DISCUSSION
The increasing trend in the number of SE cases in Ontario during the period 2005–2010 was the primary reason for undertaking this study. It is noteworthy that a similar increase in the number of SE cases occurred in the USA during the period 2004–2008 [Reference Chai24]. In Canada, a threefold increase in SE occurred from 2003 to 2009. This increase was primarily associated with PT13, PT8, and PT13a [Reference Nesbitt1].
Poultry meat
Our final multivariable model for any poultry meat consumption revealed that the odds of SE infection in individuals who consumed any poultry were more than double those who did not consume poultry. Adjusting for age did not appreciably change the estimates for any poultry meat consumption, suggesting that age is not a strong confounder. Assuming the poultry meat exposure is causal, we found that almost one-half of SE cases acquired in Ontario were attributable to consuming poultry. However, the confidence intervals around this estimate were fairly wide, reflecting a large degree of uncertainty. This relatively high population attributable fraction is due in part to the high prevalence of poultry consumption.
In the USA, chicken consumption was first identified as a risk factor for SE infections in a 1996–1997 multi-state study. More specifically, eating chicken outside the home was identified as the risk factor [matched OR (mOR) 2·0, 95% CI 1·1–3·6] [Reference Kimura14]. Eating chicken away from home was also identified as a risk factor in our univariable analysis. The US study suggests that the reason that eating chicken outside the home was identified as a risk factor is that cross-contamination from raw chicken to cutting boards, plates, and hands may be more prevalent and compounded in commercial kitchens because of greater food volumes, complex menus and undertrained food handlers. This study also noted that SE PT13a was the predominant phage type isolated from cases with domestically acquired infections who reported eating chicken prepared outside the home. SE PT13a was the second most frequently identified phage type in our study. A second US multi-state study undertaken in 2002–2003 revealed that eating chicken outside the home (OR 2·5, 95% CI 1·5–4·1) was again identified as a significant risk factor [Reference Marcus13].
The finding that consumption of poultry meat was a significant risk factor was not surprising given that SE has been isolated from chicken on farms and from retail locations. In Ontario, SE was identified on 4/68 (5·8%) commercial broiler chicken farms in 2003–2004 [Reference Arsenault25]. In 2011, SE was identified from 1/63 (1·6%) commercial broiler flocks (unpublished data, M. Guerin, University of Guelph). The phage type identified in the one positive flock was PT8. For chicken sold at retail throughout Canada, the percent of SE recovered from the Canadian Integrated Program for Antimicrobial Resistance Surveillance increased steadily from 0% (0/803) in 2003 to 5·2% (44/851) in 2009 [Reference Nesbitt1]. Sampling retail chicken from the C-EnterNet project within the Waterloo region of Ontario revealed an increase of SE from 0% (0/81) in 2005 to 4·5% (9/200) in 2009 [Reference Nesbitt1]. The increase in these numbers is consistent with the increase in human SE cases in Ontario during the same time period.
In the USA, one large study [Reference Altekruse12] conducted on broiler chicken carcass rinses showed the annual number of SE isolates increased more than fourfold and the proportion of establishments with SE-positive rinses increased nearly threefold from 2000 to 2005 (test for trend, P < 0·0001). PT13 accounted for 50% (129/257) and PT8 accounted for 35% (91/257) of the SE isolates. The authors of that study stated that the increase of SE in broiler chickens was noteworthy given the increase in human SE rates in the USA [Reference Altekruse12]. The United States National Antimicrobial Resistance Monitoring System monitoring Salmonella from chicken carcass rinses revealed that the percent of SE of all Salmonella serotypes increased steadily from ∼1% in 1997 to ∼28% in 2010 [26]. A US multi-state study noted that the increase in the percentage of chicken rinses contaminated with SE mirrored the increase in incidence in human infection. Pulsed-gel electrophoresis subtyping results also supported the link [Reference Chai24]. The relevance of these findings is that under the North American Free Trade Agreement, Canada is required to import 21% of its estimated domestic production of broiler hatching eggs as broiler hatching eggs and chicks from the USA [27].
Processed chicken
Our adjusted multivariable model for processed chicken consumption revealed that the odds of SE infection in individuals who consumed processed chicken were more than triple those who did not consume processed chicken. We found that this association was negatively confounded by age: 73% (22/30) of all cases who reported consuming processed chicken in this study were aged <20 years. This finding is consistent with other reports of salmonellosis associated with processed chicken; however, to our knowledge, this study is the first to detect an association between processed chicken and SE in non-outbreak cases. Four outbreaks of salmonellosis were reported to be associated with raw, frozen, microwavable, breaded, pre-browned, and stuffed chicken products in Minnesota from 1998 to 2006. The Salmonella serotypes included Typhimurium, Heidelberg, and Enteritidis [Reference Smith28]. In Canada, a multi-provincial, sporadic case-control study identified home-prepared chicken nuggets and/or strips as significantly associated with S. Heidelberg cases (mOR 4·0, 95% CI 1·4–13·8) [Reference Currie29]. Similarly, a study in the Canadian province of British Columbia identified frozen, processed chicken nuggets and strips as significantly associated with S. Heidelberg cases (mOR 11·0, 95% CI 1·4–85·2) [Reference MacDougall30]. Further, a Canadian study identified SE and S. Heidelberg in chicken nugget meat produced, and/or available for sale, in Canada. The two phage types identified for SE were PT13 and PT13a consistent with domestic cases identified with these phage types in our study [Reference Bucher31].
Previous studies have found that substantial proportions of processed chicken consumers do not perceive, handle or prepare these products as they would for raw, unprocessed chicken, despite the fact that many products are raw or only partially cooked [Reference Currie29]. As a result, the Canadian Food Inspection Agency's Meat Inspection Regulations were amended to state that ‘If any meat product is not a ready-to-eat meat product but has the appearance of, or could be mistaken for, a ready-to-eat meat product, the meat product shall bear the following information on its label: (a) the words “must be cooked”, “raw product”, “uncooked” or any equivalent words or word as part of the common name of the product to indicate that the product requires cooking before consumption; and (b) comprehensive cooking instructions such as an internal temperature-time relationship that, if followed, will result in a ready-to-eat meat product’ [32]. Given the findings from this study and others mentioned above pertaining to processed chicken, further research should be undertaken to evaluate the effectiveness of these labelling requirements.
Eggs
Not washing hands after handling raw eggs was identified as a risk factor in our study. Consuming undercooked eggs, and to a lesser degree eggs away from home, approached attaining statistical significance as a risk factor. A lack of study power may be a factor in not obtaining significance. Eggs have been identified as one of the main sources of SE illness [Reference Molbak and Niemann33, 34]. A 2002–2003 US case-control study revealed consuming undercooked eggs inside the home as a significant risk factor (OR 2·7, 95% CI 1·1–3·9) [Reference Marcus13]. In Canada, 300 commercial egg-laying operations were sampled in 1989 to estimate the prevalence of Salmonella. SE was isolated from the environmental samples of 8/295 (2·7%) flocks. SE PT8 was isolated from five flocks, PT13a from two flocks, and PT13 from one flock [Reference Poppe15]. In British Columbia, ungraded eggs were associated with an increase in the number of SE cases in the province between 2008 and 2010 [Reference Taylor35]. In conclusion, eggs should not be dismissed as a source of sporadic SE cases in Ontario.
Vegetarian
It is noteworthy that of the 199 SE cases, there were no vegetarians. By comparison, 7/241 controls (2·9%) were vegetarians. The fact that there were no vegetarians among the cases may be due to the absence of consuming food items that are risk factors such as poultry or eggs (in the case of vegans).
Limitations
It should be recognized that the study type is a sporadic case-control study. In contrast to outbreak investigations where there is usually one source of the illnesses, this study attempts to identify a number of possible sources for the sporadic cases. As the number of sources increases, the ability to detect the sources decreases, especially if the sources only result in a small number of cases. While this study identified food items that were associated with illness, it is likely that there are numerous sources for SE illness that were not identified. Further, contamination of food sources may be sporadic or intermittent making identification more difficult.
Bias pertaining to different interviewers administering the respective questionnaires to cases and controls is recognized; however, standardized questionnaires and interviewing protocols were expected to limit this bias. In addition, bias pertaining to the exposure histories being enquired about 3 days prior to onset of symptoms for cases and 3 days prior to the date of the interview for controls was also recognized. However, obtaining control exposure histories 2–3 weeks prior to the date of the interview, to be consistent with the cases, would have decreased the accuracy of the control data.
CONCLUSIONS
To the best of our knowledge, this is the first study identifying poultry meat consumption as a risk factor for illness due to SE in Canada. Historically in Ontario, egg consumption has been associated with illness due to SE. This study's findings reveal that consumption of poultry is a more important risk factor for SE. This is also the first study in Canada to identify processed chicken consumption as a risk factor for illness due to SE in non-outbreak cases. To our knowledge, not washing hands following the handling of raw eggs is a risk factor not previously identified in other studies.
Control measures for all of these risk factors continue to be required in order to decrease the number of human SE cases. Control measures include preventing contamination of these foods with SE as well as implementing proper food-handling practices for these foods in order to prevent illness. In addition, package labelling requirements pertaining to cooking instructions for meat products that have the appearance of, or could be mistaken for, a ready-to-eat product should be evaluated for their effectiveness.
ACKNOWLEDGEMENTS
The authors acknowledge: the Ontario Ministry of Health and Long-Term Care for providing funding for the investigation, the SE cases who consented to participate in the investigation, staff at Ontario health units for their cooperation, Ali Moterassed for performing the phage-typing work, other members of the SE Investigation Working Group including Tina Badiani, Peter Boleszczuk, Jackson Chung, Lisa Fortuna, Karen Johnson, Steven Johnson, Anne Maki, Allison McArthur, Sarah Morgan, Beata Pach, Domna Kapetanos, Duri Song, Diana Yung, and finally David Northrup at the Institute for Social Research for their involvement interviewing controls. Public Health Ontario provided funding for researchers to assist with the investigation.
DECLARATION OF INTEREST
None.






