INTRODUCTION
Toxoplasmosis, a disease resulting from infection by the protozoan, Toxoplasma gondii, can be congenital or acquired [Reference Hill and Dubey1]. T. gondii is a versatile parasite for which the prevalence of infection varies between countries, geographical areas and ethnic groups living within a specific region [Reference Tenter, Heckeroth and Weiss2]. In Brazil, infection rates range from 50% to 83% of the population depending on the location of the study [Reference Dubey3]; the high prevalence worldwide is correlated to environmental risk factors and socioeconomic factors [Reference Dubey3, Reference Baldursson and Karanis4].
There are several transmission routes with a large proportion of acquired infections related to the ingestion of cysts in raw or undercooked meat and contact with sporulated oocysts in the environment [Reference Dubey3, Reference Klaren and Kijlstra5].
Manifestations of infection by T. gondii are often subclinical, i.e. asymptomatic, or with non-specific symptoms [Reference Antoniazzi6]. Ocular toxoplasmosis (OT), associated with both congenital and acquired infections, is one of the most common manifestations of the disease [Reference Dubey3, Reference Weiss and Dubey7]. OT is the main cause of posterior uveitis, a disease that can cause serious sequelae including complete loss of vision [Reference Hay and Dutton8].
The prevalence of OT varies in different regions around the world; in Brazil about 30% of patients with eye diseases have OT [Reference Dubey3, Reference Matos9, Reference Arevalo10]. Several studies have investigated risk factors for congenital toxoplasmosis [Reference Spalding11, Reference Barbosa, Holanda and Andrade-Neto12]; however, few studies have evaluated factors related to the occurrence, severity and recurrence of OT.
The aim of this study was to investigate risk factors contributing to infection by T. gondii and to the development of OT in patients who received medical attention in an outpatient eye clinic of a tertiary teaching hospital of a public health service.
MATERIALS AND METHODS
Patient selection
Over a 2-year period (2009–2010), 349 consecutive male and female patients treated in the Outpatient Eye Clinic of Hospital de Base, Fundação Faculdade Regional de Medicina (HB-FUNFARME), a public health service in São José do Rio Preto, São Paulo state, Brazil were enrolled in this study. Each participant signed an informed consent form after receiving details about the objectives of the study and the procedures involved. This study was approved by the Research Ethics Committee of the Medical School in São José do Rio Preto, Brazil (FAMERP; no. 050/2009 dated 9 March 2009).
Epidemiological data
Participants completed a questionnaire on epidemiological data related to T. gondii infection, including social, environmental and economic factors and housing.
Diagnosis of OT
The clinical evaluation of patients was conducted by two experienced physicians (F.B.F. and G.C.A.Jr.), using an indirect binocular ophthalmoscope (Binocular Ophthalmoscope ID10, Topcon Corporation, USA). Subsequently, patients were allocated to either the OT group or the other ocular diseases (OOD) group.
Blood sampling
Serum, obtained for each patient from a peripheral blood sample collected without anticoagulant by venepuncture, was stored at −20°C until use.
Identification of IgG class anti-T. gondii antibodies
After an eye examination, IgG class anti-T. gondii antibodies were investigated by enzyme-linked immunosorbent assay (ELISA) as described previously [Reference Mattos13].
Statistical analysis
The results were analysed using GraphPad version 3.1 software (GraphPad Software Inc., USA). Fisher's exact test, odds ratio (OR) and χ 2 test were used to compare independence between proportions, and ages were compared using Student's t test. Differences were considered statistically significant at P⩽0·05.
RESULTS
The mean age of the 349 patients in this study was 56·9 ± 17·0 years (median 61, minimum 18, maximum 88 years); 187 (53·6%) were male and 16 (46·4%) were female. Ninety-eight percent (n = 342) resided in São Paulo state; 15·5% (n = 53) in São José do Rio Preto and 84·5% (n = 289) in neighbouring towns. Four patients (1·1%) resided in the state of Minas Gerais and three (0·9%) in the state of Mato Grosso do Sul. Based on the data, 73·4% (n = 256) owned a house and only 0·3% (n = 1) lived in wooden shacks.
Table 1 lists the socioeconomic parameters and environmental risk factors of patients who were seronegative and seropositive for IgG anti-T. gondii antibodies.
Table 1. Socioeconomic parameters and environmental risk factors of patients who are seropositive or seronegative for IgG anti-Toxoplasma gondii antibodies in an outpatient eye clinic, São José do Rio Preto, São Paulo state, Brazil*
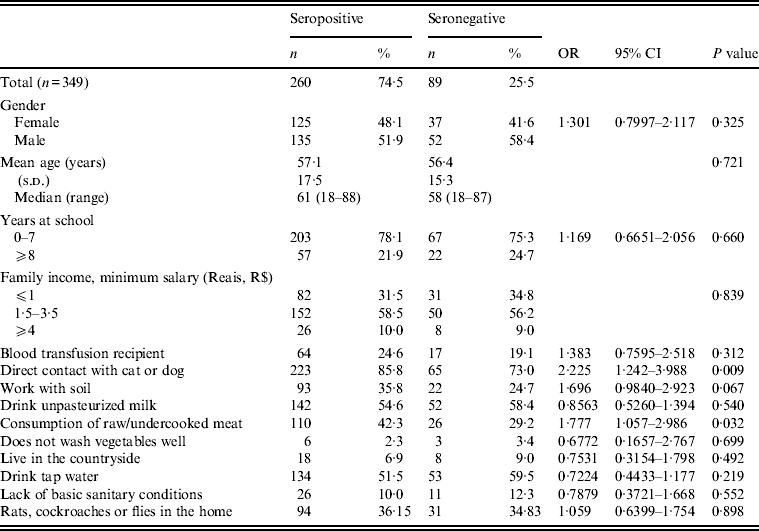
OR, Odds ratio; CI, confidence interval.
* Univariate analysis; 95% confidence interval, P⩽0·05.
Table 2 shows the socioeconomic parameters and environmental risk factors of seropositive patients with OT and with OOD.
Table 2. Socioeconomic parameters and environmental risk factors of patients seropositive for anti-Toxoplasma gondii IgG antibodies with ocular toxoplasmosis (OT) or other ocular diseases (OOD), in an outpatient eye clinic, São José do Rio Preto, São Paulo state, Brazil*
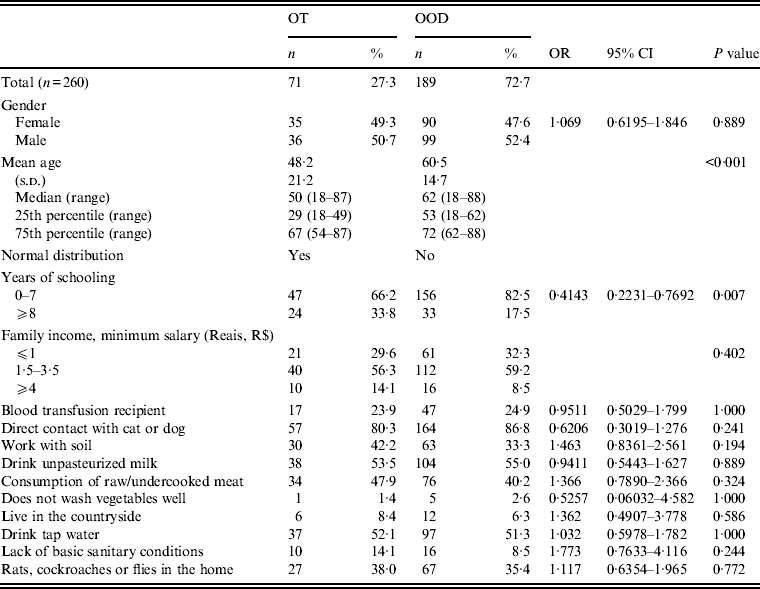
OR, Odds ratio; CI, confidence interval.
* Univariate analysis; 95% confidence interval, P⩽0·05.
DISCUSSION
This study investigated the risk factors for T. gondii infection and for the development of OT. Several studies have been conducted to ascertain the risk factors contributing to T. gondii infection in pregnant women [Reference Kapperud14–Reference Boyer16]; however, there are few studies in outpatient eye clinics that address risk factors, in particular in patients who developed OT.
ELISA was used to detect IgG anti-T. gondii antibodies as recommended, as it is frequently used to determine whether an individual has anti-T. gondii antibodies [Reference Dubey3, Reference Robert-Gangneux and Dardé17].
Patients were examined for the presence of ocular lesions by ophthalmoscopy. The high rate of patients infected by T. gondii in this study is in agreement with the literature reporting that this percentage can be as high as 80% in Brazil [Reference Dubey3], including the region of this study [Reference Gonçalves18–Reference Rodrigues20].
The frequency of OT in seropositive patients demonstrates that eye diseases caused by T. gondii are common in individuals with ocular diseases in the study region. Dubey et al. report a lower rate of ocular lesions suggestive of toxoplasmosis than that found in the present study [Reference Dubey3]. Thus the results reported here are not consistent with the findings of Dubey et al., probably due to differences in the sample population and the study region.
Indeed, the prevalence of ocular disease caused by T. gondii in Brazil varies greatly from 1·1% to 27·3% depending on the region but mainly the study population (Table 3). Most research was conducted as population-based studies, whereas in the current study the patients were selected in a referral eye clinic. Similar rates were reported by Gouveia et al. [Reference Gouveia28] in a study at a centre specializing in uveitis care in São Paulo city.
Table 3. Prevalence of ocular disease caused by Toxoplasma gondii in Brazil*
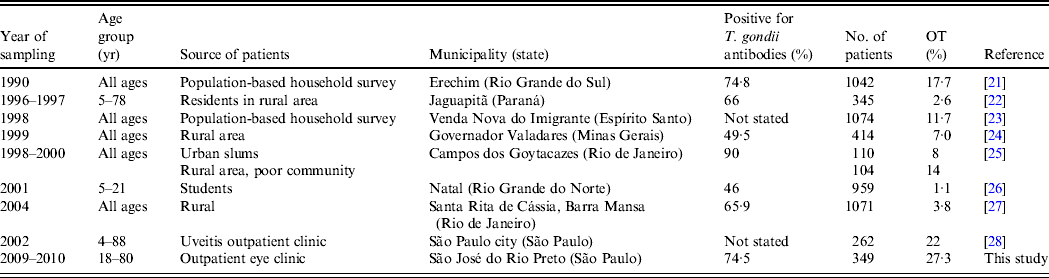
* Adapted from Table Supplement S3 of Dubey et al. [Reference Dubey3], with permission.
The parameters of age, gender, schooling and family income were evaluated in relation to the risk of infection and the development of OT. Comparing the two groups, no statistically significant differences were found between women and men. Aleixo et al. [Reference Aleixo27] found an association between ocular lesions suggestive of toxoplasmosis and women, while Jones et al. [Reference Jones29] reported a higher prevalence of OT in men, possibly due to greater contact with soil on farms and rural settings. These data suggest that the prevalence of OT by gender may be dependent on environmental and biological risk factors that were not present in our population.
No association was found between T. gondii infection and the mean age or the socioeconomic conditions, but the difference in mean age was statistically significant between seropositive patients with OT and those with OOD. Patients with OT had a lower mean age than those with OOD; OT can affect individuals at any stage of life, with 70–90% of cases being due to congenital infections and 2–30% due to infections acquired after birth [Reference Furtado30].
Additional analysis in OT patients showed lower values for the 25th percentile regarding age compared to those with OOD. This observation is consistent with previous reports and suggests that the high incidence of eye disease in congenital toxoplasmosis in Brazil causes patients to seek ophthalmology consultations early [Reference Dubey3, Reference Robert-Gangneux and Dardé17, Reference Abreu23, Reference Joynson and Wreighitt31, Reference Latkany, Weiss and Kim32].
These findings could provide some background for public policies towards maternal fetal and neonatal screening in São Paulo state and postnatal monitoring for the early identification and characterization of toxoplasmosis in children, thereby avoiding later complications and delays in the characterization of OT in children, adolescents and young adults.
In a study conducted in the High-Risk Pregnancy Clinic of Hospital de Base in São José do Rio Preto, the rate of congenital transmission in the region proved to be 2·3% [Reference Mattos33] and the acute infection rate in 556 pregnant women was 3·4% [Reference Gonçalves18].
However, there is no government programme of maternal fetal and neonatal screening in the São Paulo state nor is there postnatal monitoring for the early identification and characterization of toxoplasmosis in children, which complicates and delays the characterization of OT in children, adolescents and young adults.
There was no association between seropositive and seronegative patients and the level of schooling. A lower percentage of OT was observed in those stating that they were illiterate or had few years of schooling. This finding seems paradoxical since less schooling implies greater exposure to risk due to not adopting appropriate measures, such as good hygiene practice, to prevent infection [Reference Varella34]. It would be expected that individuals with a higher level of schooling would have a lower risk of developing OT. It is possible that individuals with better schooling go to eye clinics more frequently and therefore the diagnosis in this group is higher.
The association observed in this study between infection with T. gondii and direct contact with cats and dogs has been reported previously [Reference Barbosa, Holanda and Andrade-Neto12, Reference Lopes35]. This observation is plausible because the cat is the definitive host of the parasite and thus eliminates oocysts in stools, which after maturation, contaminate the environment [Reference Dubey, Lindsay and Speer36]. It is believed that consumption of canine meat may contribute to the spread of the parasite in humans, as has been demonstrated in other diseases [Reference Wertheim37]; however, Brazilians do not have the habit of eating canine meat. However, there is the possibility of contamination due to oocysts in dog fur, as well explained by Frenkel & Parker [Reference Frenkel and Parker38].
It was noted in the current study that infection is strongly associated with the consumption of raw or undercooked meat, a habit believed to be the main infection route of T. gondii. An association was also found in this study, albeit insignificant, between T. gondii infection and regular contact with soil. Direct contact with earth/soil suggests that infection may also occur in this way [Reference Spalding11, Reference Cook39]; and industrialized processed meat and poor hygiene habits also contribute to infection with T. gondii [Reference Bojar and Szymanska40–Reference Alvarado-Esquivel42].
No association was found with other factors evaluated and T. gondii infection. No statistically significant differences were observed when environmental risk factors associated with T. gondii infection were analysed in relation to the development of OT. As the risk factors for infection are not associated with OT, it is possible that the risk of developing OT is dependent on the pathogenicity of the infecting strain and/or the immune competence of the host coupled with immunogenetic factors that determine susceptibility. However, these factors are inherent in patients and the parasite requires further study.
In conclusion, the results of this study confirm that the presence of dogs and cats, as well as the consumption of raw or undercooked meat increases the risk of infection, but does not influence the development of OT.
ACKNOWLEDGEMENTS
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (L.C.M., no. 2009/17540-2) (C.S.M., no. 2009/09168-6) (V.L.P.C., no. 2011/13939-8) (F.N. no. 2012/07750-2) (C.R.B., no. 2012/05580-2); Brazilian Ministry of Science, Technology and Inovation–CNPq (L.C.M., no. 473579/2009-0); Brazilian Ministry of Education–CAPES Scholarship (A.I.C.F, C.C.B.M, C.R.B); and BAP-FAMERP (L.C.M).
The authors thank J. P. Dubey and colleagues, for sharing the data in Table 3, previously published in reference [Reference Dubey3]. Thanks are also due to: Dr Lilian Castiglioni (Department of Public Health; FAMERP) for statistical analysis support, and David Hewitt and Jim Henson (AcademicEnglishSolutions.com) for English revision. The authors also thank the Biotechnology Research Institute of Auckland University of Technology, New Zealand, particularly Professor Stephen Henry.
DECLARATION OF INTEREST
None.





