Introduction
Despite recent improvements following the successful implementation of the Millennium Development Goals, tuberculosis (TB) is still the leading cause of mortality due to a single infectious pathogen [1].
The transmission of infectious diseases is affected by characteristics of the environment, infectious agent, and host, including both the infecting and infected hosts. For directly transmissible diseases, understanding the characteristics of the index patient that influence the capacity to transmit infection is essential to design the effective control strategies [Reference Woolhouse2, Reference Galvani and May3]. The spread of TB is strongly affected by the infectious individual's characteristics, with some individuals causing considerably greater numbers of new infections, while others produce very few or none [Reference Wang4].
A central goal of TB control strategies is the prevention of transmission. Hence, identifying highly contagious TB patients, by analysing risk factors from contact investigation studies, is critical for designing effective and efficient TB control interventions. We performed a systematic review of patient factors associated with the risk of transmission of TB to contacts.
Methods
The review protocol was prospectively registered with PROSPERO (International Prospective Register of Systematic Reviews, registration number: CRD42016053913) and we adhered to the guidelines of Meta-analyses and Systematic Review of Observational studies in Epidemiology (MOOSE) throughout [Reference Stroup5].
Search strategy
We searched the following electronic bibliographic databases from inception to 3 January 2017: MEDLINE, EMBASE, Web of Science, Global Health and Global Health Archive. All search terms were ‘exploded’ to include all resources and consisted of ‘Tuberculosis’, ‘Mycobacterium tuberculosis’, ‘TB’, ‘contact’, ‘contact investigation’, ‘transmission’, ‘index case’, ‘infectiousness’, ‘risk factors’, ‘secondary case’ and ‘latent infection’. The detailed search strategy is provided in Table S1.
Study selection
Search results were exported to EndNoteX8 (Clarivate Analytics, NY, USA) and duplicates were removed. Titles and abstracts were screened to identify potentially relevant articles. The outcome of interest was latent TB infection (LTBI) or TB disease among contacts of index TB patients. The full texts of the studies that were deemed relevant in this initial screening phase were then obtained and reviewed by two authors (Y. M. and T. D.). Studies were included if they diagnosed index patients according to the clinical, radiological or microbiological criteria, assessed LTBI or TB disease among contacts, reported the number of index patients and contacts, and quantified index patient-related risk factors for infection or disease in their contacts. Editorials, commentaries, case reports, outbreak reports, systematic reviews, mathematical modelling studies and studies in languages other than English were excluded, as were studies that did not assess index patient-related risk factors.
Data extraction
The following data were extracted: country, year, study design, index patient definition, contact definition, outcome status of contacts (LTBI or TB disease), number of index patients, number of contacts, study population, contact screening methods, results of screening and index risk factors investigated. Estimates of the effect of exposures on the outcomes of the proportion of contacts with active TB or with LTBI were also extracted, including adjusted risk ratio or odds ratio and their 95% confidence intervals (CIs). Y. M. and T. D. extracted the data independently using the same data extraction form with all discordant data resolved by consensus.
Assessment of study quality
Each article was evaluated using the Newcastle–Ottawa Scale [Reference Wells6] for its validity with respect to the selection of study participants, comparability between exposure groups and outcome measurements, by two authors (Y. M. and T. D.) (Table S2).
Meta-analyses
Exposure variables were selected based on the availability of sufficient data and were not specified a priori, three variables were chosen for meta-analysis based on importance and consistent availability: sputum smear-positivity, lung cavitation and HIV seropositivity. Meta-analyses were performed with MetaXL (Version 5.3, EpiGear International Pty Ltd, QLD, Australia) to estimate the pooled effects of each of these three exposures on the binary outcomes of contact LTBI or TB disease. Random effects models were used to estimate pooled effect measures. Heterogeneity among estimates was quantitatively evaluated with the I 2-statistic.
Results
Search results
The search strategy resulted in 4780 references, of which 1725 were duplicates, 321 were deemed eligible for full-text review and 37 studies were included in the systematic review. The detailed flow diagram of study selection is presented in Fig. S1. Of the included studies, 17 were cross-sectional, seven cohort studies, 12 follow-up studies without control groups and one case–control study. Twenty-four studies originated from one of the 30 high burden TB countries.
Contact and index definitions
Studies varied by the types of index patients and contacts included. Eight defined index patients as newly diagnosed smear-positive pulmonary TB patients, 12 studies considered confirmed pulmonary TB and six included all types of TB. With regard to contact definition, 20 studies considered only household contacts, six studies traced only children and adolescent contacts and three considered only close contacts who shared enclosed spaces (Table S3).
Outcome measures
The outcome of interest was LTBI or TB disease amongst contacts of different index patients, which was used as a marker of patients’ infectiousness; that is the capacity to transmit Mycobacterium tuberculosis (M. tb) to contacts. The included studies compared contacts who developed the outcome (LTBI or TB disease) to those who did not, based on the characteristics of index patients as the exposure variable. Thus, the measures of association (risk ratio or odds ratio) quantified the effect of exposure to an index patient with a given characteristic on the probability or odds of infection or disease in the exposed contact. The majority of included studies (29/37) considered LTBI as the main outcome, with 25 using tuberculin skin test (TST), one using interferon-gamma release assay (IGRA) and three using both TST and IGRA to determine infection. All studies using IGRAs to define infection used the manufacturer's recommended cut-off assessed at a single time point to define infection. Studies using TST mostly used a cut-off ⩾10 mm for HIV negative and ⩾5 mm for HIV positive contacts, although six studies used a cut-off of ⩾5 mm for all contacts and two other studies used 15 mm as the cut-off point for children having BCG vaccination. The remaining eight studies used the development of active TB in contacts as the measure of transmission (Table S4).
Index patient risk factors for M. tb transmission
The included studies considered a range of characteristics of index patients for their association with transmissibility. We categorised these index risk factors as demographic, behavioural, co-morbidities, TB-related clinical and household characteristics. We preferentially used the adjusted association reported from multivariate analyses. We also classified studies based on the type of association they had reported as: positive, negative or non-significant based on a P-value of <0.05 or a 95% CI not including unity.
Sociodemographic risk factors
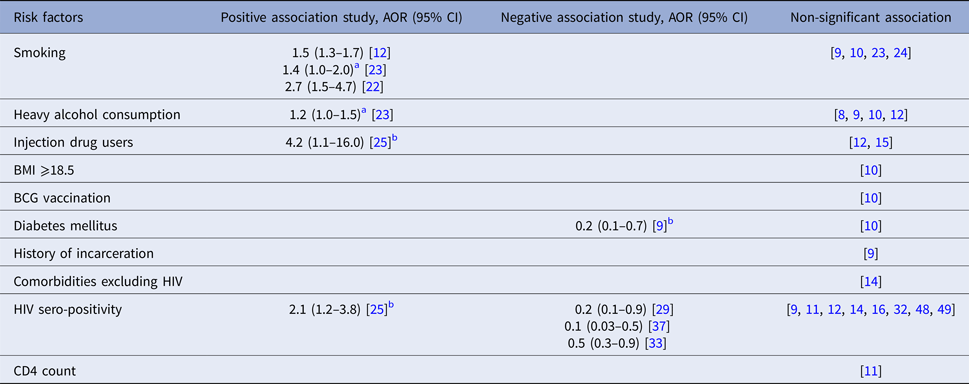
Sex of index patients was a non-significant factor in most studies reporting its association with transmissibility [Reference Otero7–Reference Snider17], although two studies found female sex to be associated with greater risk of infection [Reference Kifai and Bakari18, Reference Tornee19]. With one exception [Reference Lohmann13], included studies considered adult index patients exclusively. Of these, most studies that reported the effect of age found a non-significant association [Reference Otero7, Reference Grandjean9, Reference Godoy12, Reference Grandjean14, Reference Golub15, Reference Elliott20], although two studies reported that increasing age was positively associated with M. tb transmission [Reference Snider17, Reference Madhi21]. Of these two studies, one was conducted in France among contacts aged under 17 and found that contacts of patients aged 40 years old or above were more likely to be infected than contacts of younger patients [Reference Madhi21]. By contrast, a study from the USA reported that contacts of patients aged 64 years or above were less likely to be infected than contacts of younger patients [Reference Snider17].
One study from the Netherlands, which considered both close and casual contacts found that contacts of index patients who were employed or attending school were more likely to be infected [Reference Lohmann13], while two studies from Peru considering household contacts only found that employment status was not a significant risk factor for M. tb transmission [Reference Grandjean9, Reference Grandjean14]. Black race of the index patient was a positive risk factor in one study in the USA which considered all close contacts [Reference Golub15], while another study of contacts aged under 15 years in the USA found race was not associated with contact infection [Reference Snider17] (Table 1).
Table 1. Socio-demographic risk factors for infectiousness of index patients extracted from included studies
a Confidence interval not provided.
b Adjusted relative risk.
c Considered as a categorical variable.
AOR, adjusted odds ratio; CI, confidence interval.
Behavioural factors and co-morbidities
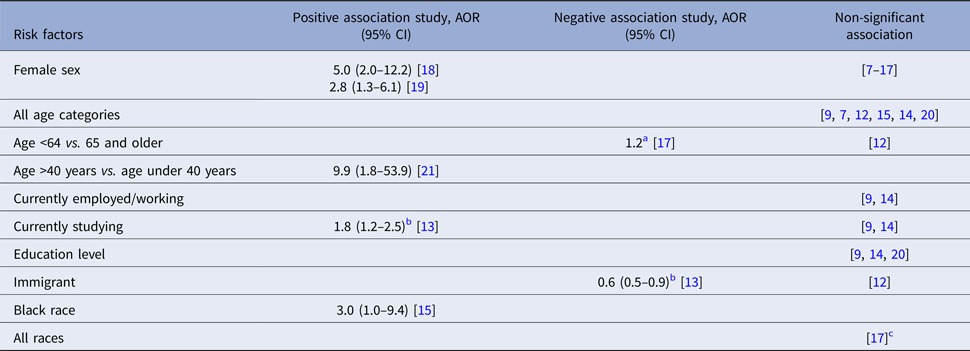
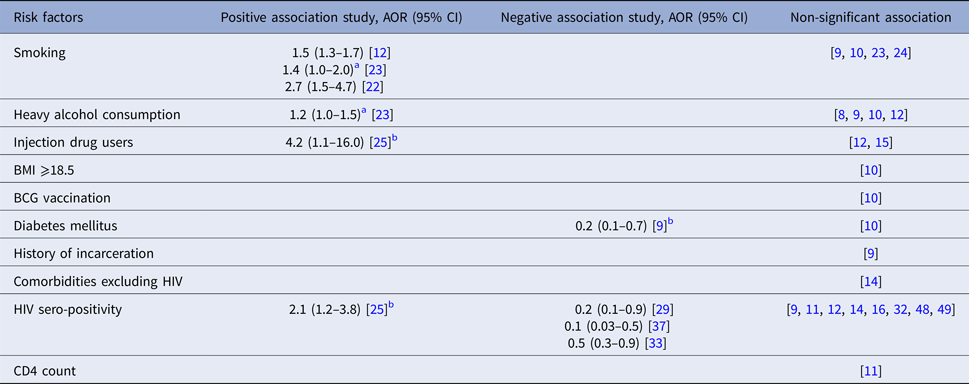
Index patients’ smoking status was a significant risk factor for LTBI among contacts in three studies [Reference Godoy12, Reference Singh22, Reference Huang23], but was non-significant in four [Reference Grandjean9, Reference Faksri10, Reference Huang23, Reference Nair24]. In one study from Peru, smoking more than one cigarette per day was associated with TB transmission to child contacts, but was not significant for transmission to adults. This study also reported that heavy alcohol consumption (compared with no alcohol intake) was associated with risk of contact infection [Reference Huang23], although other studies [Reference Jones-Lopez8–Reference Faksri10, Reference Godoy12] found no significant association. Similarly, injection drug use was associated with higher TB transmission in a case–control study from Spain [Reference Cayla25], but this risk factor was non-significant in others [Reference Godoy12, Reference Golub15] (Table 2).
Table 2. Behavioural and co-morbidity risk factors for infectiousness of index patients extracted from included studies
a Adjusted relative risk.
b Indicates that these studies take contact active TB disease as an outcome.
AOR, adjusted odds ratio; CI, confidence interval.
TB-related clinical risk factors
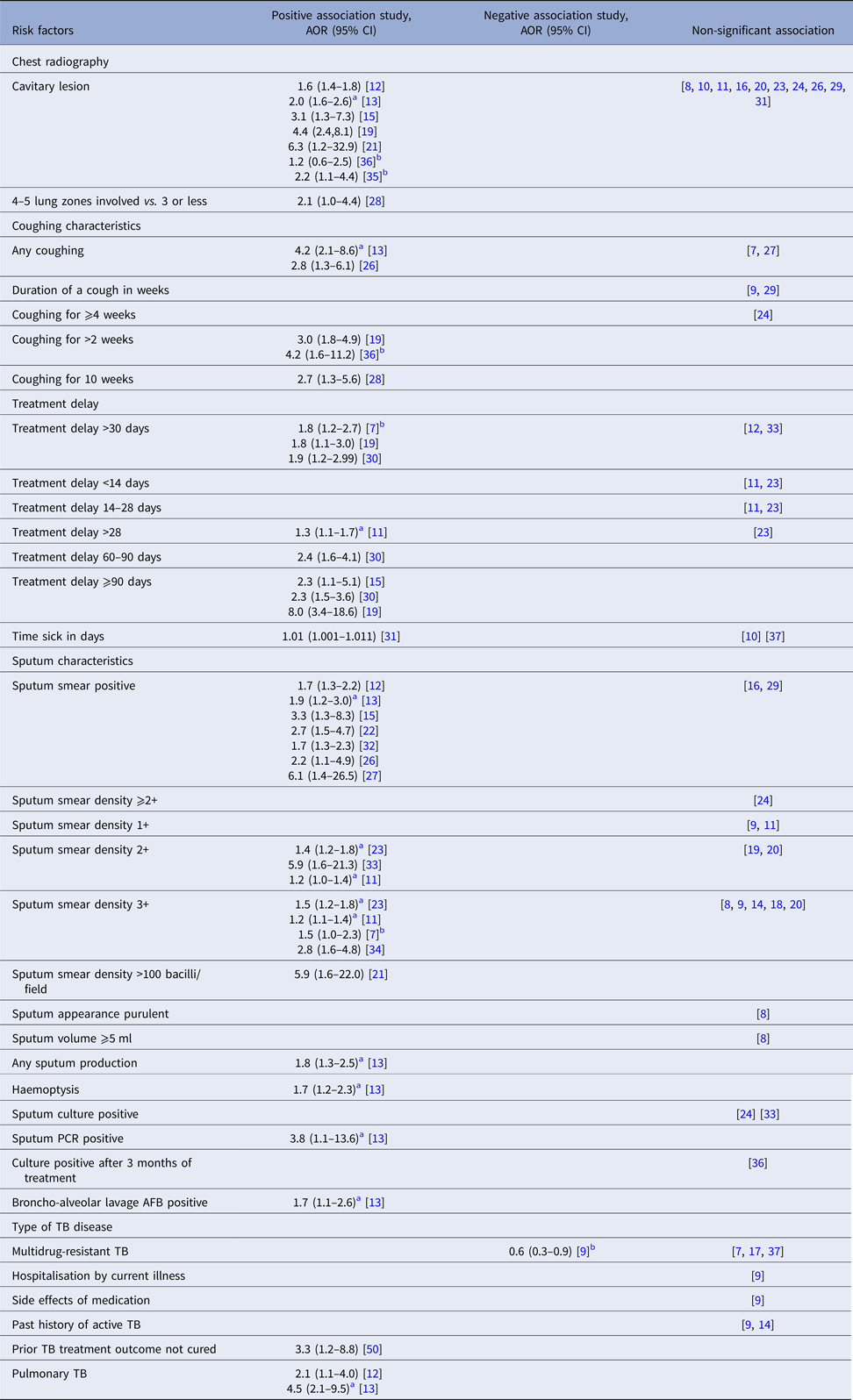
Differences in the index patients’ clinical presentation were considered as potential risk factors for M. tb transmission to contacts by several studies (Table 3). Compared with contacts of index patients who did not cough, contacts of index patients with any cough were more likely to be infected in two studies [Reference Lohmann13, Reference Gessner, Weiss and Nolan26], while another two studies found no significant association [Reference Otero7, Reference Elmi27]. Of studies considering the duration of coughing, two studies found that contacts of patients coughing for 2 weeks or longer were more likely to be infected than contacts of patients who coughed for a shorter period of time or who did not cough [Reference Lohmann13, Reference Tornee19], while a study reported coughing for 5 weeks or longer to be a significant risk factor [Reference Lienhardt28]. In contrast, three studies found that duration of a cough, considered as a continuous variable was not statistically significant [Reference Grandjean9, Reference Nair24, Reference Mohammad29].
Table 3. Index tuberculosis disease-related clinical risk factors for infectiousness of index patients extracted from included studies
a Adjusted relative risk.
b Outcome considered was active TB disease in contacts.
AOR, adjusted odds ratio; CI, confidence interval.
Treatment delay was another risk factor considered for M. tb transmission, although studies considered different cut-off points for categorising a number of days of treatment delay. In three studies [Reference Otero7, Reference Tornee19, Reference Xu30] a treatment delay of 30 days or more (compared with <30 days) was associated with greater risk of infection and disease among contacts. Similarly, compared with no treatment delay, delay for more than 28 days [Reference Huang11] was a risk factor in one study, while in two other studies [Reference Golub15, Reference Xu30] delay of 90 days or more was significant. Another study reported the duration of illness considered as a continuous variable to be a significant factor for contact LTBI [Reference Mendes31].
Sputum smear-positivity was reported to be associated with M. tb transmission by ten studies [Reference Godoy12, Reference Lohmann13, Reference Golub15, Reference Singh22, Reference Gessner, Weiss and Nolan26, Reference Elmi27, Reference Loredo32]. Other studies compared patients based on the bacilli density per field, although categorisation was inconsistent. Sputum smear bacilli density of 2+ [Reference Huang11, Reference Huang23, Reference Espinal33] and 3+ [Reference Otero7, Reference Huang11, Reference Huang23, Reference Rathi34] compared with scanty were significant risk factors in all studies reporting this variable. Similarly, one study [Reference Madhi21] reported very high sputum smear densities ⩾100 bacilli/field as a significant risk factor. Sputum production and haemoptysis were found to be significant risk factors in one study [Reference Lohmann13].
Lung cavity lesion findings were associated with greater infectiousness in eight studies [Reference Godoy12, Reference Lohmann13, Reference Golub15, Reference Tornee19, Reference Madhi21, Reference Guwatudde35, Reference Lee36], but non-significant in another nine studies [Reference Jones-Lopez8, Reference Grandjean9, Reference Huang11, Reference Nunn16, Reference Elliott20, Reference Huang23, Reference Nair24, Reference Gessner, Weiss and Nolan26, Reference Mohammad29, Reference Mendes31]. The number of lung zones involved on CXR was also reported as a risk factor in one study, with the involvement of four or more zones associated with more contact infections than those with fewer zones involved [Reference Lienhardt28].
Of the four studies which compared transmission based on drug sensitivity, one [Reference Grandjean9] reported contacts of multidrug-resistant TB (MDR-TB) patients were less likely to be infected, while three [Reference Otero7, Reference Snider17, Reference Teixeira37] reported no significant difference.
Household risk factors
A history of TB among family members was found to be a risk factor for contact TB disease in two studies [Reference Nair24, Reference Lee36], although this was non-significant in one more recent study [Reference Otero7]. The type of residence of the index patient was also reported as a significant factor, with those living in slums or apartments associated with more infections among contacts [Reference Tornee19]. Household crowding with two or more persons per room was a significant risk factor for M. tb transmission in one study [Reference Tornee19] although two other studies found no significant effect [Reference Nair24, Reference Mohammad29].
Meta-analysis
Three factors were reported in a sufficient number of studies to permit meaningful meta-analysis. Twenty-six studies were included in one or more of these meta-analyses, with three factors of interest being sputum smear-positive disease, lung cavitation and HIV status of the index patient (Table S5).
Contacts of sputum smear-positive pulmonary TB patients were found to be more frequently infected than contacts of smear-negative patients, with a pooled adjusted odds ratio (AOR) of 2.15 (95% CI 1.47–3.17, I 2 = 38%) (Fig. 1). A similar association was observed from the pooled crude odds ratios (2.29, 95% CI 1.84–283, I 2 = 54%) (Fig. S2). Sensitivity analysis indicated that no single study, when excluded, affected the pooled estimates (Table S6), although funnel plots suggested possible publication bias (Fig. S5).
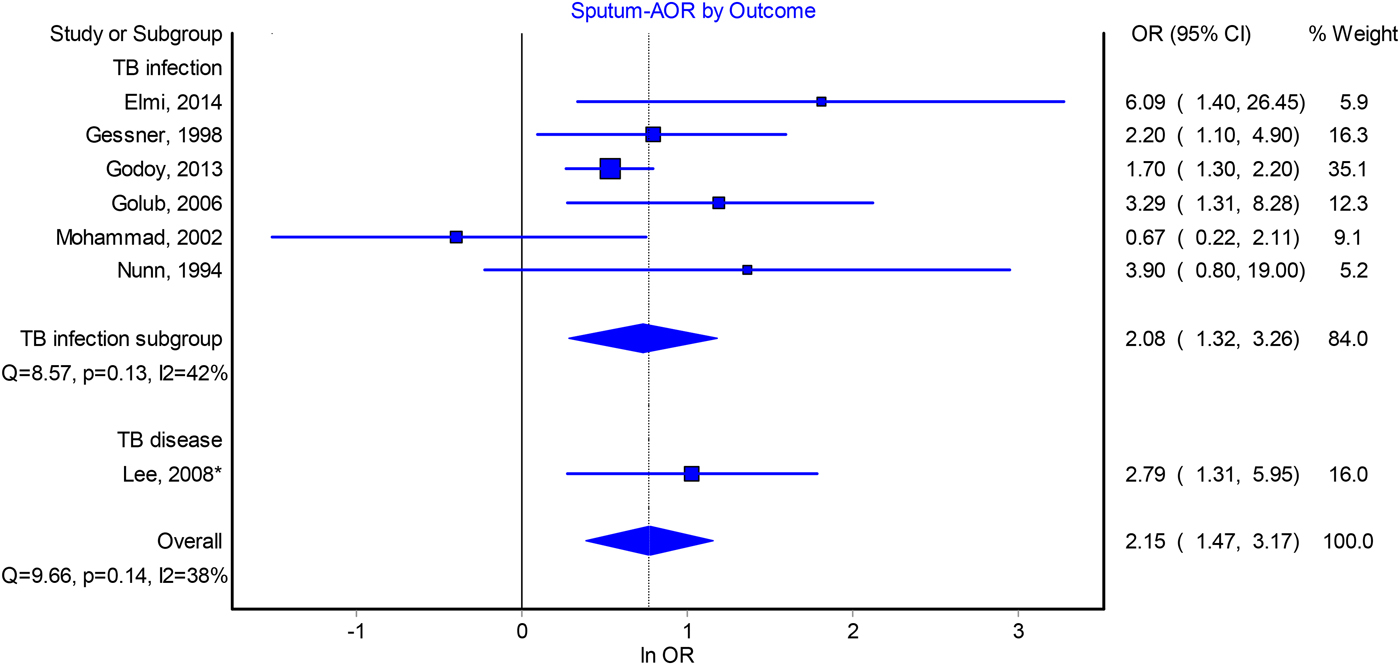
Fig. 1. Forest plot of adjusted odds ratios for the association between index patient sputum smear-positivity and infectiousness.
The odds of infection among contacts of patients with cavitation also appeared to be around two, although heterogeneity between study estimates was substantial. Our estimate of the pooled AOR is 1.90 (95% CI 1.26–2.84, I 2 = 63%) (Fig. 2) and 1.93 (95% CI 1.36–2.75, I 2 = 85%) for the pooled crude odds ratio (Fig. S3). In sensitivity analyses, excluding a cross-sectional study from Thailand [Reference Tornee19] lowered the pooled AOR to 1.64, while excluding other studies did not markedly change our point estimate. In the crude odds ratios analysis, exclusion of any individual study did not result in a marked change to our pooled estimate (Table S7) and publication bias was not revealed by funnel plots (Fig. S6).
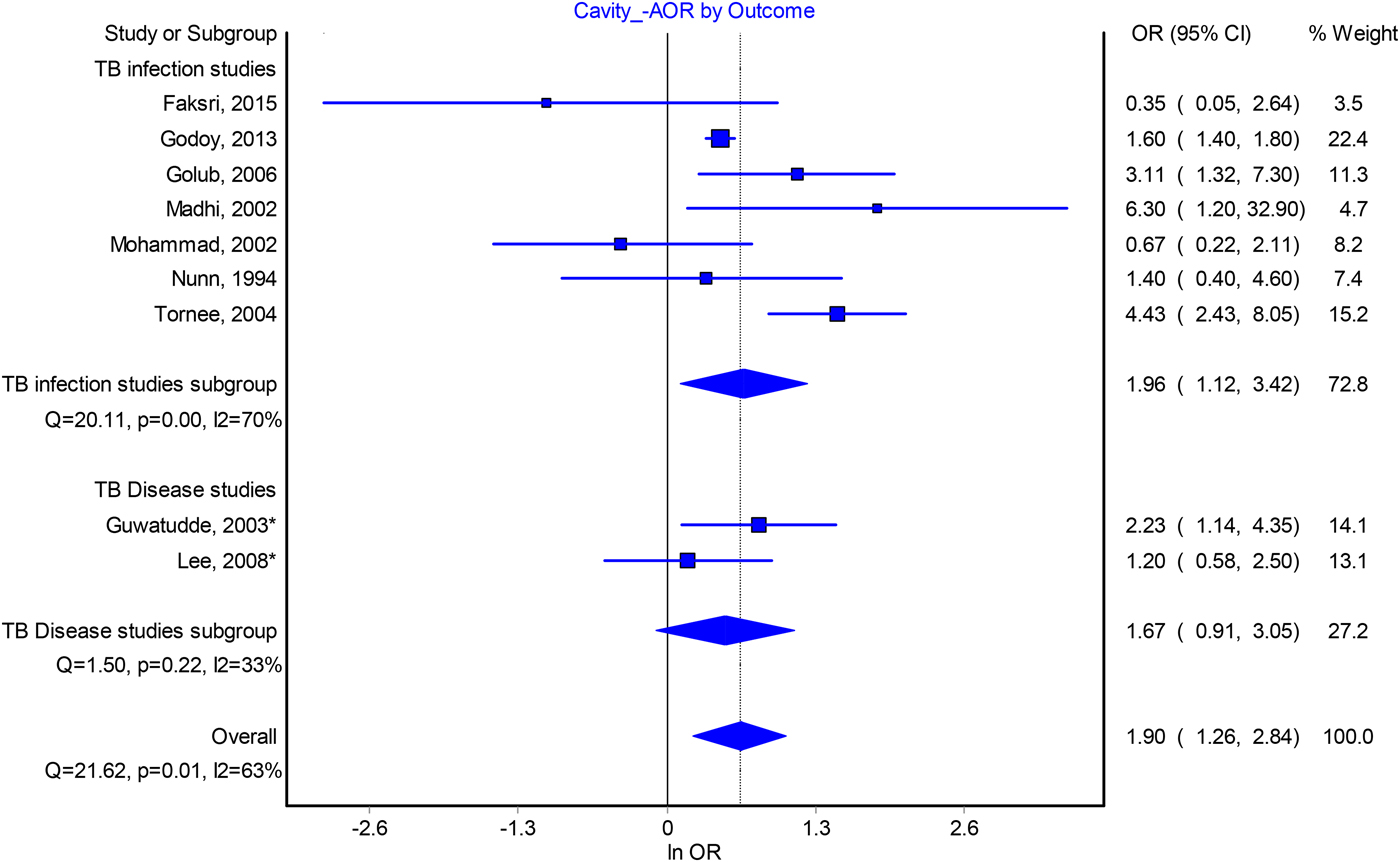
Fig. 2. Forest plot of the association between index patient lung cavitation and infectiousness from adjusted odds ratio analysis.
HIV seropositivity of index patients was negatively associated with infectiousness in the AORs analysis (0.45, 95% CI 0.26–0.80, I 2 = 52%) (Fig. 3). However, this association was non-significant in the analysis of crude odds ratio (0.76, 95% CI 0.55–1.07, I 2 = 80%) (Fig. S4). Sensitivity analysis of the adjusted estimate found that excluding any one of the studies did not change the pooled estimate significantly (Table S8) and the funnel plot of studies revealed no evidence of publication bias (Fig. S7).
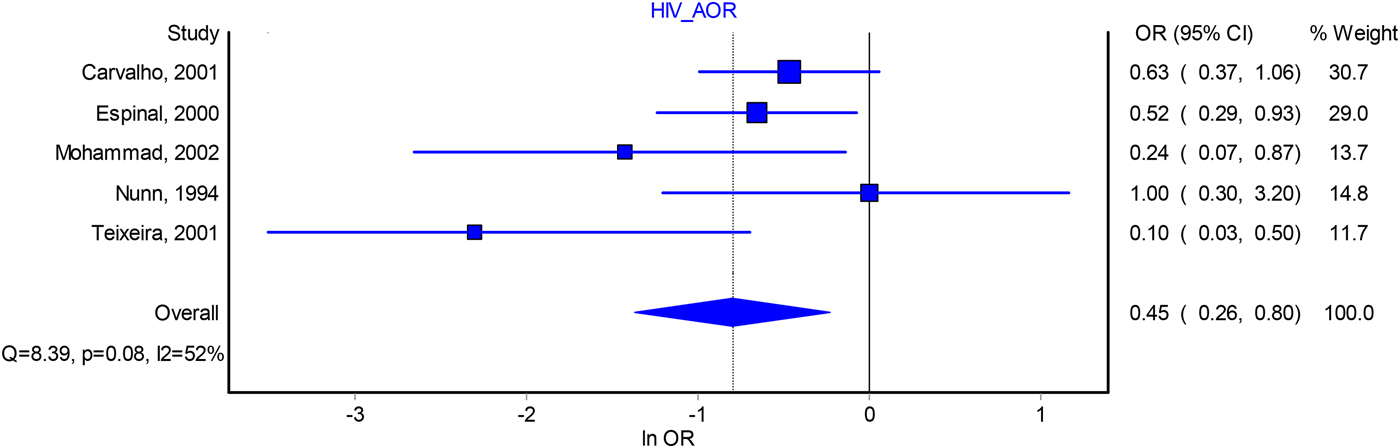
Fig. 3. Forest plot of the association between index patient HIV seropositivity and infectiousness from adjusted odds ratio analysis. *Indicates that these studies take contact active disease as an outcome. AOR, adjusted odds ratio; OR, odds ratio; CI, confidence interval.
Discussion
This systematic review and meta-analyses assessed the capacity of index TB patients to transmit the infection to their contacts based on their demographic, behavioural and clinical characteristics. Our meta-analysis showed that sputum smear-positivity, the presence of lung cavitation and HIV seronegativity were the factors most consistently associated with greater infectiousness of index cases. Our results were also broadly consistent with the premise that more severe lung parenchymal involvement being associated with greater infectiousness. We found no sociodemographic characteristics that were consistently associated with the infectiousness of patients. Clinical factors were studied and reported in a variety of ways, but generally consistent with the message that prolonged symptoms such as coughing and delay to diagnosis lead to increased infectiousness. Treatment delays of >28 days were found to be a predictor of a greater proportion of contact infections.
Although most studies found no significant association, smoking and alcohol intake were found to be risk factors for greater infectiousness in some studies. A possible explanation could be that these risk factors are associated with both biological changes in the host [Reference Davies38], as well as differences in social mixing behaviours such as sharing enclosed bars [Reference Lönnroth39], such the association is likely to be setting specific. In addition, exposure to second-hand smoke increases the susceptibility of contacts to infection, particularly among children [Reference Jubulis40, Reference Du Preez41].
Compared with smear-negative patients, smear-positive patients were found to be approximately twice as infectious. This association was not confounded by cavitation since all studies but one included in the pooled analysis had adjusted for this factor [Reference Elmi27]. The positive association between smear-positivity and infectiousness is likely to reflect smear-positive patients expelling a greater number of M. tb into the environment than smear-negative patients, as M. tb bacilli are only detectable with sputum microscopy when the concentration of the bacilli is sufficient [Reference Initiative42]. Similarly, index patients presenting cavitation were found to be twice as infectious as those without cavitation. This is consistent with the widely held belief that cavitary TB is the consequence of lung tissue damage and reflects the development of macroscopic openings between the site of infection and the airways, which facilitate expectoration of the bacteria [Reference Ernst43].
In contrast, we found that patients living with HIV were around half as likely to transmit to their contacts, after adjusting for other factors. Notably, all such studies involved patients with untreated HIV. A previous meta-analysis found no significant difference between HIV negative and positive index patients with regards to their infectiousness, although it should be noted that this study only pooled crude odds ratios or relative risks [Reference Cruciani44], in contrast to our study which considered estimates adjusted for potential confounders. The lower infectiousness of HIV-positive index patients may be due to their less frequent cavitation (due to suppression of the immune response) [Reference Ernst43, Reference Haramati, Jenny-Avital and Alterman45] or patterns of social mixing. Of the five studies included in this analysis, two [Reference Nunn16, Reference Mohammad29] adjusted for cavitation and one of which found HIV status non-significant [Reference Nunn16]. HIV-positive patients may also be diagnosed and treated for active TB more rapidly due to fast disease progress or their closer contact with health care services, which may decrease their infectious period. It has previously been found that HIV-positive TB patients are less likely to be smear positive than HIV-negative TB patients [Reference Elliott46, Reference Klein47]. However, four of the five studies included in our meta-analysis adjusted for sputum smear status, such that the association of HIV seropositivity with TB transmission does not appear to be mediated by sputum smear-positivity. Therefore, HIV-positive patients may have a modestly reduced level of infectiousness but should not be presumed to be non-infectious, which is consistent with extremely high disease burden in high HIV-burden countries. As such, an equal focus of contact investigation has been advised for both HIV-seropositive and negative index patients [Reference Martinez48].
The funnel plots suggest possible publication bias, particularly for studies that examined the association of infectivity and smear positivity. Other potential sources of bias result from the observational nature of studies included in this review, including selection bias and information bias. Non-differential misclassification bias could arise due to the inability of TST to differentiate recent LTBI attributable to the index exposure from distant past infection. This would dilute the effect, leading to an under-estimate and a bias towards the null. This may particularly be seen in studies with broader definitions of contacts, where distant latent infection might represent a greater proportion of all latent infection. By contrast, studies employing stricter definitions and focusing on those with greater exposure to the index case, such as close contacts only, would provide more accurate estimates, although this may be offset by lower precision due to smaller sample sizes. Studies using stricter definitions of infection (e.g. higher TST cut-offs) would also obtain more accurate effect size estimates, but at the expense of precision. Duration of follow-up differed between studies, with some assessing their study group at only a single point in time, which may affect the development of an infection response and its detection. Such insufficient duration of follow up could similarly lead to underestimation of the outcome measures, through greater dilution from distant infection. Studies considering less infectious index patients, such as those that included smear-negative patients, could also have a greater proportion of disease attributable to remote infection and a bias towards the null.
We have reviewed the outcomes of infection and subsequent disease together under the implicit assumption that both of these outcomes reflect index patient infectiousness. This is only correct if we consider that contacts have a similar risk of reactivation following infection regardless of the characteristics of the index patient. We believe this is reasonable because the risk of reactivation should be primarily attributable to contact characteristics (e.g. age, comorbidities) and because pooling of studies considering active disease with those considering LTBI did not increase statistical heterogeneity, although it could also be influenced by characteristics of the organism and extent of exposure. However, we have carefully indicated which outcome is being considered throughout and presented disaggregated meta-analysis results. Last, it should be noted that we are considering infectiousness as the proportion of contacts infected or diseased and have not considered the absolute number of infections generated, which may also reflect infectiousness to some extent.
Conclusions
We identified three clinical characteristics that can be used to identify TB patients with a greater capacity for transmission; smear positivity, the presence of cavitation on chest X-ray and HIV status. Thus, in an era of increasing use of molecular diagnostics, traditional diagnostic methods, such as sputum smear microscopy and chest radiography, still provide important information on patients’ infectiousness. HIV-positive TB patients not under treatment may be modestly less infectious, although such patients should not be presumed to be non-infectious. Sociodemographic and behavioural characteristics were less consistently associated with infectiousness and their importance may be setting-dependent. The public health implication of these findings is in the potential to provide close follow-up and targeted interventions for these highly infectious TB patients.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817003041
Acknowledgements
The authors thank the Monash University for providing access to electronic databases. Y. A. Melsew is a recipient of a Monash Graduate Scholarship for his PhD study. No specific funding was received for this study.
Declaration of Interest
The authors declare that they have no competing interests.