INTRODUCTION
Community-acquired pneumonia (CAP) is associated with significant morbidity and mortality. It ranks as the third leading cause of death worldwide [Reference Lopez and Murrey1] and is one of the most common infectious diseases in the Western industrialized countries [Reference Welte, Marre and Suttorp2]. Its annual incidence varies between 1·6 and 11·6/1000 inhabitants [Reference Jokinen3–Reference Almirall5]. In Germany the annual incidence is estimated to be around 10 cases/1000 inhabitants [Reference Welte, Marre and Suttorp2]. According to the Federal Statistical Office in Germany 18 395 people died in 2004 from CAP, representing 2·2% of all fatalities.
Smoking, alcohol consumption, increasing age, chronic pulmonary disease and other chronic underlying diseases such as diabetes, cirrhosis or cardiac failure were reported as risk factors for CAP [Reference Almirall5, Reference Almirall6–Reference Farr9]. However, most of the studies were limited either to patients in defined settings such as hospitals or long-term care facilities [Reference Farr10, Reference Loeb11], or they determined specific exposures such as metal fume or alcohol consumption [Reference Palmer12, Reference Fernández-Solá13]. To our knowledge there are no large epidemiological studies to date which determine risk factors for CAP in the German adult population. We conducted a population-based, case-control study in order to identify the main risk factors for CAP in Germany. When sampling cases, we took advantage of the CAPNETZ competence network and compared the cases included in CAPNETZ with population-based, random controls. CAPNETZ was initiated in June 2002 by a grant from the Federal Ministry of Education and Research and accumulates data from in-patients and outpatients with CAP to combine diagnostic, therapeutic and epidemiological research strategies for CAP in adults. We investigated whether known risk factors such as smoking, frequent previous respiratory infections, chronic pulmonary diseases, and other chronic underlying diseases [Reference Kohlhammer14], were also relevant to the situation in Germany. Furthermore, we hypothesized that a parental history of CAP, contact with pets and number of children in the household may also play a role as risk factors for CAP.
MATERIAL AND METHODS
Study population
Cases were sampled from the CAPNETZ competence network [Reference Welte, Marre and Suttorp15, Reference Bauer16]. During the study period it incorporated 11 local clinical centres (LCC) throughout Germany which collaborate with surrounding sentinel practices and hospitals. Eight university hospitals, 24 secondary hospitals and around 600 practices reported new CAP cases to the study monitor of the corresponding LCC. The study monitor decided whether to include the patient in CAPNETZ by applying the following criteria: age >18 years, an infiltrate diagnosed by chest X-ray and, in absence of this, clinical symptoms consisting of a temperature ⩾38·3°C (rectal) and cough or purulent sputum, or positive auscultation. Criteria for exclusion were an acquired or therapeutically induced immune deficiency, florid tuberculosis or an infection of possible nosocomial acquisition (hospitalization within 4 weeks prior to infection). After inclusion in CAPNETZ, the patient's medical history and clinical parameters were assessed in a standardized fashion and entered into a database. Additionally, the study monitor distributed a self-administered questionnaire which collected information on potential risk factors. No limits of age or health status were applied to distribution, but only patients who were mentally and physically able to complete the questionnaire received our questionnaire. The recruitment period for our analysis was 1 June 2002 until 30 April 2005.
The control group was randomly selected from the population of Luebeck and Magdeburg. A sample of 1000 persons was drawn from each registration office. The controls were frequency matched by sex and age (stratified by age in ten-year intervals) to the distribution of the CAPNETZ population on 6 August 2003. In early November 2003, a revised questionnaire was sent to these 2000 people including general information about the study. A reminder including another copy of the questionnaire was sent in December 2003 and, if necessary, again in January 2004. We took into account all questionnaires which were returned by 31 March 2004.
All patients and controls gave informed consent to participation, and the study protocol was approved by the ethic committees of the participating universities.
Questionnaire
The questionnaire given to the cases comprised information about the actual case history, previous history of CAP, parental history of CAP, the presence of upper respiratory infections in the previous year, the presence of hay fever or difficulties swallowing, influenza and pneumococcus vaccination status, consumption of coffee, tea and alcohol, lifetime smoking history, physical activities, occupational and non-occupational environmental exposure to noise, dust and contact with metal, living conditions at home (number of children aged <15 years, number of people living in the home, exposure to pets), and socio-demographic data. The questionnaire also included questions on general and health-related life satisfaction. Body mass index (BMI) and chronic underlying diseases such as chronic pulmonary disease, chronic heart disease, chronic renal disease, diabetes and chronic liver disease were assessed by the reporting physician.
The questionnaire given to the controls was an identical subset of the CAP cases' questionnaire with respect to the following items: past history of CAP, parental history of CAP, the presence of upper respiratory infections in the previous year, the presence of hay fever or difficulties swallowing, influenza and pneumococcus vaccination status, lifetime smoking history, physical activities, living conditions at home, socio-demographic data and questions on life satisfaction. In addition, the questionnaire included medical history, self-reported height and weight and questions on stress as well as social support.
Statistical analysis
Data analysis was performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences between cases and controls were analysed by the χ2 test for categorical variables. For the comparison of means, the non-parametric Mann–Whitney U test was used for continuous variables which were not normally distributed. Otherwise the Student's t test was used. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated. Multivariate analysis of factors potentially associated with CAP was performed by stepwise logistic regression taking CAP (case/control) as dependent variable and including all parameters which showed statistical significance in univariate analysis as independent factors. A P value of <0·05 was considered statistically significant.
To determine whether our results were representative of all CAPNETZ cases we compared the responders with the group of non-responders by age, sex, place of treatment (outpatient/in-patient) and severity of CAP measured by the ‘CRB-65’ scoring system (1 point is given for each feature present: confusion, respiratory rate ⩾30/min, blood pressure (SBP <90 mmHg or DBP ⩽60 mmHg) and age ⩾65 years) [Reference Capelastegui17]. The risk of death increases with an increasing CRB-65 score. Severe CAP is defined as a CRB-65 score >2 [Reference Bauer18, Reference Lim19].
RESULTS
Response rate and non-responder analysis
In the recruitment period 3402 CAP cases were included in CAPNETZ. Of these, 2084 cases received the questionnaire and 1137 questionnaires were returned, which is equivalent to a response of 54·6%. The proportion of outpatients was 39·1%. Overall, 98·2% of the cases were radiographically confirmed (3·2% of the outpatients and 0·4% of the in-patients were not diagnosed by chest X-ray). Cases diagnosed by clinical symptoms were younger then cases radiographically confirmed (mean±s.d.: 50·7±16·7 vs. 57·1±17·1, P=0·09) and the proportion of patients with chronic respiratory diseases was significantly lower (11·8% vs. 33·3%, P=0·046).
The comparison between the responders and non-responders showed that a pathogen could be identified in 37·6% of the responders and in 37·0% of the non-responders. The most frequently identified pathogens were Streptococcus pneumoniae (responders 35·7%, non-responders 31·9%), Legionella spp. (responders 10·9%, non-responders 11·2%), influenza A virus (responders 7·7%, non-responders 9·4%), Mycoplasma pneumoniae (responders 33·9%, non-responders 34·9%), and Haemophilus influenzae (responders 2·9%, non-responders 2·1%). The patient's actual health status was the main reason for not distributing the questionnaire (57·1%). Compared with the cases who received a questionnaire this group was significantly older and hospitalized more frequently (Table 1). In addition, the proportion of patients with severe CAP was significantly higher (10·8% vs. 1·1%, P<0·001) in the group which did not receive a questionnaire. Other reasons for not distributing the questionnaire were logistic problems (17·3%) or poor understanding of German (7·4%).
Table 1. Description of the CAPNETZ group, the excluded group, responders and non-responders

* Differences were statistically significant between excluded cases and cases receiving the questionnaire (P<0·001).
† Differences were significant between non-responders and responders (P<0·05).
The main reasons for refusals given by patients were no interest in participating in the study (30·5%), actual health status (20·9%), doubts about the purpose of the study (9·0%), and language and time problems (5·1% each). The non-responders did not differ significantly from the responders except for the proportion of men (Table 1).
From the control group 1044 persons returned completed questionnaires resulting in a response rate of 53·4%. In Luebeck 578 persons returned the questionnaire, 39 persons dropped out because of death, moving house or other reasons, which led to a response rate of 60·1%. Out of the 1000 persons contacted in Magdeburg, 466 completed the questionnaire and 13 dropped out because of death or moving house, which yielded a response rate of 47·2%.
Demographics
Demographic data are given in Table 2. Compared with the control group there were no significant differences as regards age, sex, health insurance or marital status. Only for school education did the case group differ significantly from the control group. Nearly half of the cases had finished school after ⩽9 years, a quarter finished after 10 years and another quarter after ⩾12 years. Similarly, in the control group a quarter had finished school after ⩾12 years, but the proportion of controls which finished school after 10 years was significantly higher than in the case group.
Table 2. Demographic data of cases and controls

* P<0·05.
Risk factors for CAP
Factors which showed an association with CAP in univariate analysis are shown in Table 3. Previous CAP obviously determines the risk of actual infection. Other risk factors related to CAP were a history of more than one respiratory infection in the previous year and hospitalization in the last 5 years. Chronic pulmonary, renal, liver and heart diseases were also associated with CAP. The higher the number of underlying diseases, the higher was the risk of CAP. Our analysis also confirmed smoking as a risk factor. Furthermore, the risk of CAP increased with the number of children living in the household. Compared with normal weight, persons who were underweight showed an increased risk of CAP while being overweight seemed to be protective.
Table 3. Univariate analyses of potential risk factors for community-acquired pneumonia (CAP)
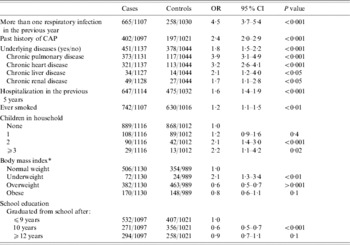
OR, Odds ratio; CI, confidence interval.
* Cut-off points are defined by 20·1, 25·1 and 30·1 for males and 19·1, 24·1 and 30·1 for females.
Neither parental history of CAP nor exposure to a pet was significantly associated with CAP. Hay fever or difficulties in swallowing were also not related to the acquisition of CAP. Furthermore, living with a partner, living in a nursing home and number of hours of physical activity were not associated with the risk of CAP in this analysis (data not shown).
The results of the logistic regression analysis are presented in Table 4. A previous CAP, more than one respiratory infection in the previous year, chronic pulmonary disease, an increasing number of underlying chronic diseases, being underweight and an increasing number of children present in the household remained independent risk factors for CAP. Smokers also had an increased risk of CAP compared to never-smokers (not significant). Being underweight remained a stable risk factor and overweight remained a protective factor.
Table 4. Multivariate analysis of risk factors for community-acquired pneumonia (CAP)

OR, Odds ratio; CI, confidence interval.
* Cut-off points are defined by 20·1, 25·1 and 30·1 for males and 19·1, 24·1 and 30·1 for females.
We included sex and age in a further multivariate model. The effect estimates, however, changed only marginally (data not shown). The multivariate model stratified by sex showed that women were more strongly affected than men with respect to exposure to children in the household (Table 5). A significant association between CAP and children was found only in men living with two children in the household, but in all women living with one or more children.
Table 5. The effect of children in the household stratified by sex
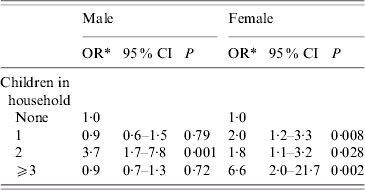
OR, Odds ratio; CI, confidence interval.
* Adjusted for the variables included in Table 4.
A further stratified analysis by age showed that an increasing number of children in the household remained a risk factor for CAP in people aged ⩽65 years (2 children: OR 2·2, 95% CI 1·4–3·4; ⩾3 children: OR 2·9, 95% CI 1·3–6·4). In the >65 years age group the case numbers were too small to give a conclusion which was statistically reasonable.
DISCUSSION
Our study provides, to our knowledge, the first assessment of risk factors for CAP in a representative German adult population. The results of our study suggest that previous CAP, repeated upper respiratory infections, chronic pulmonary diseases, an increase in the number of underlying chronic diseases, smoking and being underweight were independent risk factors for CAP. These findings are comparable with those of previous studies. Almirall et al. found in a population-based study in Spain, that people with a previous diagnosis of pneumonia had a two-fold higher risk of subsequent CAP [Reference Almirall6]. The risk was progressively higher for recent episodes compared to those longer ago. Jackson et al. reported in a study of seniors enrolled at a health maintenance organization, that hospitalization for pneumonia in the year before the start of the study was associated with CAP (OR 1·98, 95% CI 1·64–2·40) [Reference Jackson20]. Repeated upper respiratory infections and chronic pulmonary diseases as risk factors for CAP were also identified in previous studies [Reference Almirall6, Reference Farr9, Reference Farr10, Reference Koivula, Sten and Mäkäla21, Reference Gutiérrez22]. In addition, smoking is a well-known and important risk factor for CAP. Possible mechanisms, by which smoking increases the risk of CAP, include structural changes in the respiratory tract such as peribronchiolar inflammation, increased mucosal permeability and a decrease in the immune system [Reference Arcavi and Benowitz23]. Several studies have shown that smokers have a two-fold risk of CAP [Reference Farr10, Reference Almirall24]. Nearly 30% of the overall risk is attributable to cigarette smoking [Reference Jackson20, Reference Almirall24]. Almirall et al. reported that, among adults who quit smoking, the excess risk of CAP appears to decrease 5 years after quitting [Reference Almirall24]. In our study we observed a higher risk for smokers, which did not remain statistically significant in the multivariate analysis. This effect might result from the smaller groups and the higher variability in the regression model. Moreover, there might be an association between ‘chronic pulmonary disease’ and smoking, which affected our results. It is known that smoking is also a risk factor of chronic pulmonary disease such as chronic obstructive pulmonary disease (COPD) [Reference Wilson25–Reference Johannessen27] and asthma [Reference Gwynn28]. Smoking might promote the development of chronic pulmonary diseases and this in turn, might contribute to the development of CAP.
Low body weight has been previously related to pneumonia possibly because of malnutrition and underlying illness [Reference Almirall6, Reference Baik8, Reference Riquelme29]. The protective impact of overweight in our study is in contrast to other findings. Almirall et al. did not find such an association [Reference Almirall6]. Baik et al. observed in a study of age and lifestyle factors in relation to CAP that a BMI of 27·0–29·9 was a risk factor for CAP (OR 1·87, 95% CI 1·26–2·77) in women [Reference Baik8]. It is plausible that this is due to increased respiratory resistance resulting from reduced lung volume [Reference Jubber30] or to the development of atelectasis resulting in a change in lung structure [Reference Koenig31]. In our study we observed that overweight persons have a reduced risk of CAP (OR 0·6, 95% CI 0·5–0·7). A possible explanation for this finding could be a misclassification bias due to difficulties in interpreting X-ray photographs of overweight persons. It is likely that some overweight cases were not diagnosed and were not included in CAPNETZ, which results in an underestimation of the risk. Another explanation could be that BMI is based on self-report of height and weight by the controls. It is known that self-reported values of anthropometric data are less accurate than clinical measurements [Reference Gunell32]. Short people tend to over-report their height and overweight tends to be under-reported, resulting in a biased estimate of the association between BMI and CAP. Further research is needed to clarify these conflicting results.
In our study we found a strong association between the number of children in the household and an increased risk of CAP, which followed a dose–response relation. The risk of CAP increased with the number of children in the household. The most likely explanation is an increased exposure to infectious agents. This assumption was confirmed by the stratified analyses, which showed that women, who might spend more time at home than men, were more strongly affected than men. In addition, we did not find a significant effect from children in the household in people aged ⩾65 years. However, this could be explained by an infrequent exposure in this age group. In addition, few studies showed that the use of pneumococcal vaccine in young children prevents pneumococcal disease in adults [Reference Whitney33, Reference Lexau34]. This indicates that the pneumococcus bacterium, the most important causative pathogen of CAP [Reference Farr10, Reference Jackson20, Reference Riquelme29], is transmitted from children to adults. Further evaluation is needed of the extent to which crowding plays a role in CAP. In a study comparing seniors admitted to hospital for CAP and population-based controls, no significant differences in crowding, defined as the number of persons living in the home divided by the number of rooms, were found [Reference Farr10]. In populations such as Brazilian children living in slums or inmates of US jails, crowding was identified as a risk factor for CAP [Reference Fonseca35, Reference Hoge36].
Our study has strengths and limitations. We consider the population basis of cases and controls and the inclusion of in-patients and outpatients as strengths of our study. The few population-based studies have either smaller sample sizes [Reference Almirall6] or were restricted to the elderly [Reference Jackson20, Reference Koivula, Sten and Mäkäla21]. We avoided the selection procedures applied in other studies, which restricted the sample to hospitalized patients or other selected groups. It can be assumed that our results are more generalizable to the general population. Most of the cases (98·2%) were diagnosed by chest X-ray, so that there was certainty concerning the diagnosis. Other strengths were the standardized definition of CAP in our study and the use of standardized, identical instruments when collecting data. We assessed several risk factors which had, as yet, not all been investigated, such as living conditions including the number of children at home, exposure to pets or parental history of CAP. In addition we conducted a non-responder analysis in the case group. A pairwise matched case-control design usually demands a matched analysis. This is not necessarily the case in frequency-matched studies like this. We did, however, compare multivariate models with and without adjustment for sex and age, but the effect estimates remained largely unchanged.
A limitation of our study is that the controls were recruited only from the population of Luebeck and Magdeburg, while cases originate in many regions in Germany. Assuming that the population of Luebeck represents West Germany and Magdeburg represents the former East Germany, any potential effect due to selection might be limited. However, a comparison between the cases from Luebeck and Magdeburg and the cases from the other nine regions did not yield any difference regarding age, sex and severity of CAP.
The high proportion of non-responders in the case group is another limitation of our study. Only 61% of the CAP cases received a questionnaire. The study monitor excluded CAP patients on grounds of age or a CRB-65 score >2. In addition, 46% of the cases who received a questionnaire did not return it. This differential participation resulted in a younger subpopulation of CAP cases in our analysis and a lower proportion of hospital-recruited cases and therefore, potentially, in a lower proportion of cases with a CRB-65 score >2. The magnitude and direction of this effect can be assessed, however, since the CRB-65 score of responders and non-responders can be compared.
In our study, univariate analysis showed an increase in the risk of CAP for chronic underlying diseases such as pulmonary, liver and renal diseases, but in the multivariate analysis only chronic pulmonary disease remained statistically significant. This may have been due to the low prevalence of these diseases and the limited statistical power to detect such an association. Further, self-reported chronic underlying diseases in controls were compared with chronic underlying diseases reported by a physician. Self-reporting could result in less reliable information and may be affected by underreporting. This could lead to an overestimation of the effects. Finally, case-control studies are always prone to recall bias. This, however, may affect the investigated factors in a different way. It can be assumed that cases recall parameters which are associated with pulmonary symptoms in a better way than controls. However, the number of children living in the household should be recalled in a similar way in both groups.
The association between school education, as proxy for the social status, and the development of CAP, was inconsistent. Graduating from school after 10 years was protective (OR 0·6, 95% CI 0·5–0·8) compared with completing school education after 9 years, while graduating from school after ⩾12 years did not show an effect. We cannot conclude, therefore, that a lower social status independently increases the risk for CAP.
In summary, the present study identified, besides known risk factors such as previous CAP, repeated respiratory infections, chronic underlying pulmonary diseases and underweight, the number of children in the household as risk factor of CAP. A better understanding of the association between the presence of children and the acquisition of CAP is needed to develop successful health promotion strategies for adults.
APPENDIX. The CAPNETZ Study Group
Markus Becker, Antje Kuhnke, Hartmut Lode, Malina Schmidt-Ioanas, Norbert Suttorp (Berlin), Torsten Bauer, Santiago Ewig, Barbara Schlosser (Bochum), Matthias Pletz (Hannover), Klaus Dalhoff, Sven Pischke, Niels Schübel (Lübeck), Ingrid Huntemann, Joachim Lorenz (Lüdenscheid), Tom Schaberg, Konstanze Voigt (Rotenburg), Martin Hetzel, Philipp M. Lepper (Ulm), Berthold Jany, Uwe Ziegler (Würzburg), Torsten Illmann, Michael Wallner, Michael Weber (IT), Heike von Baum, Susanne Gonschior, Klaus Richter (main office) and all the study nurses.
ACKNOWLEDGEMENTS
This study was supported by the German Federal Ministry of Education and Research [Bundesministerium für Bildung und Forschung (BMBF)], by grants 01KI0103-105.
DECLARATION OF INTEREST
None.







