INTRODUCTION
Foodborne illness is an important health concern in many countries. In the USA there are an estimated 76 million cases of foodborne illness annually, affecting 25% of the population and leading to over 325 000 hospital admissions and 5000 deaths [Reference Mead1]. Comparable morbidity, hospitalization and mortality rates are reported in England and Wales [Reference Adak, Long and O'Brien2]. A number of organisms contribute to foodborne illness, with Campylobacter spp., Salmonella spp., Clostridium perfringens, Verocytotoxin-producing Escherichia coli (VTEC) O157, and Listeria monocytogenes being the most significant [Reference Adak, Long and O'Brien2].
Increased ambient temperature may lead to increased foodborne illness for several reasons. First, under certain conditions some bacteria, such as Salmonella spp., multiply in food in direct proportion to temperature, within the range 7·5–37°C. In the absence of any control measures increased ambient temperatures may therefore increase bacterial reproduction at various points along the food chain, making the consequences of any subsequent ingestion more severe [Reference Heyndrickx3–Reference Tam, Rodrigues and O'Brien5]. Second, ambient temperature may influence people's behaviour, which in turn may affect the chance of a foodborne illness occurring. For example, increased temperature may lead to elevated consumption of raw foods such as fruit and salad (at risk of cross-contamination), and higher temperatures may encourage riskier cooking practices such as barbecuing. Finally, warmer temperatures may lead to increased outdoor recreational activity which may make it more likely that people will be exposed to environmental sources of the relevant gastrointestinal pathogens. Although these illnesses are not strictly ‘foodborne’, routine surveillance data cannot readily distinguish between these illnesses and those which are foodborne. However, it is estimated that the majority of campylobacteriosis and salmonellosis cases are foodborne in origin [Reference Adak, Long and O'Brien2].
Consequently, it is unsurprising that many studies have demonstrated positive associations between temperature and foodborne illness in a variety of geographical settings. These include England and Wales [Reference Bentham and Langford6], Europe [Reference Kovats4], China [Reference Zhang7], Peru [Reference Checkley8], the Pacific Islands [Reference Singh9] and Australia [Reference D'Souza10]. These studies have been utilized to explore the aetiology of disease, and by other authors to examine the potential health impacts of climate change [Reference McMichael, Woodruff and Hales11, Reference Ebi12].
None of these studies have considered whether the relationship between temperature and foodborne illness has changed over time. This is worth investigating as, subsequent to the completion of many of these studies, there have been significant changes in the trends of foodborne illness. Figure 1 presents the number of food poisoning notifications together with reported cases of campylobacteriosis, salmonellosis and the two main serovars of Salmonella spp., S. Enteritidis and S. Typhimurium in England and Wales. The data demonstrate that after many years of high incidence during the 1990s the number of reported cases has started to decline. Similar reductions in foodborne illness have been observed in the USA [13] and the European Union [14]. A re-consideration of the relationships between foodborne illness and temperature, and whether these have changed over time, is therefore overdue.

Fig. 1. England and Wales weekly foodborne illness notifications and laboratory reports 1974–2006.
Data
This paper examines the impact of temperature upon the weekly counts of foodborne illness in England and Wales. The first analysis focuses upon food poisoning notifications obtained from the Office for National Statistics and the Health Protection Agency (HPA) from 1974 to 2006. Food poisoning notifications are statutory notifications of any disease of an infectious or toxic nature caused by, or thought to be caused by, the consumption of food or water [Reference Wall15]. This diagnosis can be made in the absence of laboratory investigation/confirmation, leading to some outcome uncertainty. The main advantage to using food poisoning notifications is the exceptionally long time-series available for analysis (1974–2006). Each case is dated according to when it was reported to national surveillance.
The analysis then focused upon specific organisms responsible for foodborne illness. The weekly numbers of all laboratory-confirmed cases of non-typhoidal salmonellosis (1981–2006) and campylobacteriosis (1989–2006) reported to national surveillance were obtained. Illness with typhoidal salmonellas (S. Typhi and S. Paratyphi) was excluded from the analysis because these organisms have a different epidemiology, and in England and Wales are usually travel related [16]. The salmonellosis data were also subdivided into illness from the two main serovars S. Enteritidis and S. Typhimurium because these organisms have different transmission pathways and consequently may display different relationships with temperature. Each case was dated according to the ‘earliest specimen date’. This is a calculated date using (as available) the date when the stool specimen was taken, the date the specimen was received at the laboratory where the sample analysis was undertaken or the date the sample result was reported to national surveillance. All cases where the individual had reported recent foreign travel were excluded from the analysis as the infection may have been acquired abroad. This removed 15·2% of salmonellosis cases, 13·1% of S. Enteritidis infections, 9·1% of S. Typhimurium infections and 4·1% of campylobacteriosis cases. This distinction was not available for food poisoning notifications. Individuals notified as food poisoning cases may be investigated further by laboratory analysis of a faecal sample, and the majority of salmonellas and most campylobacters will be reported by both means [Reference Atkinson and Maguire17]. In these cases they appear in both the food poisoning notifications and the laboratory-confirmed cases of a specific illness reported to surveillance. However, other pathogens such as Cryptosporidium and Giardia are commonly reported as food poisoning.
Mean temperature for each week was obtained from the Central England Temperature series. This is a weighted mean temperature for England and Wales derived from measurements at four representative meteorological stations within the area of central England. These data provide a good representation of average national conditions [Reference Parker, Legg and Folland18]. Temperature was calculated for the same week as the foodborne illness and up to 8 weeks previously. The length of any significant lags provides insight into the point at which contamination of the foodstuff occurred or existing contamination multiplied. Short-term lags with temperature indicate effects occurring close in time to when the food was consumed. Long-term lags are indicative of effects further back in the food production process. Lags also allow us to account for the delay between a foodborne illness occurring and a stool sample being taken or the case being reported (in the case of food poisoning).
In order to control for possible reporting artefacts in the data, we created a dummy variable to define weeks containing a public holiday. These periods can lead to under-reporting as a patient may be less able to visit their doctor. A dummy variable was also defined for weeks following a public holiday when there may be over-reporting of cases due to patients, unable to visit their doctor in the previous week, making their visit in the subsequent week. There is evidence that many foreign travel cases are not distinguished in national surveillance. Therefore, for each week the number of reported foreign travel cases of campylobacteriosis, salmonellosis, S. Enteritidis infection and S. Typhimurium infection were obtained from national surveillance for each illness. These were used as a marker of under-reporting. Such data were not available for food poisoning notifications.
METHODS
The weekly counts of food poisoning, campylobacteriosis, salmonellosis, S. Enteritidis infections and S. Typhimurium infections were first adjusted for effects that could bias the results. Figure 1 demonstrates that all the foodborne illnesses demonstrated long-term trends in incidence. These may be due to real changes in incidence or alternatively changes in reporting rates over time. These long-term trends were eliminated by taking the natural logarithm of each weekly incidence as a dependent variable, and fitting a polynomial of time for the period, in a regression analysis. Higher-order polynomials of time were fitted until the addition of further terms did not improve the model fit. The food poisoning data was detrended with a sixth-order polynomial of time, the campylobacteriosis and salmonellosis data with a fourth-order polynomial of time and illness with S. Enteritidis and S. Typhimurium with fifth- and sixth-order polynomials of time, respectively. The residuals from these models were taken as the detrended time series for further analysis. An additional problem is that seasonal differences in food poisoning incidence may not be dependent on temperature. For each foodborne illness, we took the detrended residuals and subtracted the mean residual incidence for that week (i.e. every week 1, week 2, …, week 53) to produce detrended, deseasonalized residuals. These were used as the dependent variables in all subsequent analyses. The temperature data were also deseasonalized by subtracting the mean temperature for that week (for every year there was corresponding foodborne illness data), to produce deseasonalized temperature.
The analysis of the five health outcomes proceeded in stages. First, the weekly detrended and deseasonalized health incidence data was used as the dependent variable. This was then included in an ordinary least-squares regression analysis including the deseasonalized temperature in the current and up to 8 previous weeks as explanatory variables. In order to control for possible data artefacts, all these models included the two public holiday dummy variables. They also included the number of reported travel cases for each illness that week, detrended and deseasonalized as per the dependent variable. This analysis indicated that many of the temperature variables were positively and significantly associated with the health outcome measures. However, due to significant collinearity between the temperature variables, and the fact that this would increase when the interactions between temperature and time were considered, the temperature variables were grouped into two time periods. Temperature in the current and previous week (T c,p) was the mean for the same week plus 1 week earlier, when any effects of factors operating close to the point of consumption would be most evident. Temperature 2–5 weeks previously (T 2–5 wk pr) was the mean temperature for 2–5 weeks earlier when factors operating during food production, processing and distribution might be more important [Reference Bentham and Langford6]. The results indicated that temperature >5 weeks away was not important. In order to explore whether the impact of temperature had changed over time, these new models also included interaction terms between the two temperature variables and the sequential time variable (i.e. T c,p×time, T 2–5 wk pr×time). All the variables controlling for data artefacts (public holiday in the current week, public holiday in the previous week, and the number of reported travel cases of each illness) were also included in the models.
In order to ensure the robustness of the results all models were fitted with and without an autocorrelation term indicating the health outcome variable in the previous week. A different method for detrending the data by regressing the health outcome data against indicator dummy variables for each year in the series was also tested. Finally, to account for known long-term changes in temperatures, the analyses were duplicated by detrending the temperature variables using a linear term. All the results presented in the paper were unaffected.
RESULTS
The results for each of the five foodborne illnesses are presented in Table 1. All explanatory variables were included in every model, but in the salmonellosis and the S. Enteritidis infection model the interaction variable between T 2–5 wk pr and time was highly collinear with the other variables leading to unstable relative risks and significance levels. It was consequently excluded.
Table 1. Ordinary least-squares regression model of foodborne illness and temperature
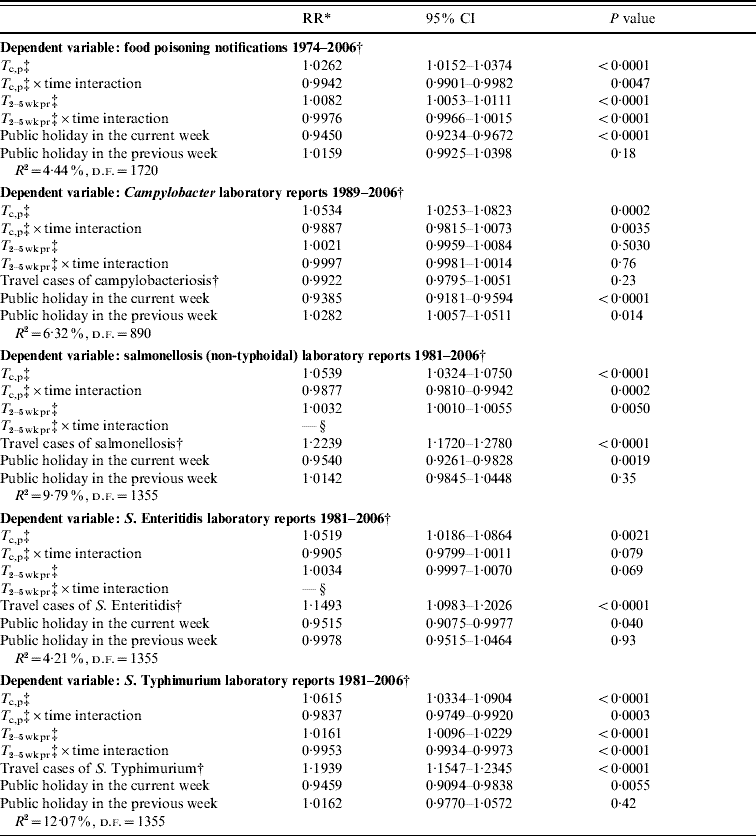
T 2–5 wk pr, Temperature 2–5 weeks previously; T c,p, temperature in the current and previous week; RR, relative risk; CI, confidence interval.
* Proportional change in risk per unit change in dependent variable.
† Detrended and deseasonalized.
‡ Deseasonalized.
§ Omitted due to severe collinearity with the other explanatory variables.
The results demonstrate that the risks of all five causes of illness were positively associated with T c,p. For food poisoning, campylobacteriosis, salmonellosis and S. Typhimurium infection the T c,p×time interaction was also significantly associated with a lowering of risk indicating that the influence of temperature has declined over time. For S. Enteritidis infection this interaction variable is also <1, but not significant (P=0·079).
The influence of T 2–5 wk pr showed a less clear pattern. It is significantly and positively associated with the risk of food poisoning, salmonellosis and S. Typhimurium infection. T 2–5 wk pr approaches significance (P=0·069) for S. Enteritidis infection but is insignificant for campylobacteriosis. For food poisoning and S. Typhimurium infection the T 2–5 wk pr×time interaction term was significantly associated with a decreased risk, providing strong evidence that the importance of T 2–5 wk pr has diminished over time. For salmonellosis the T 2–5 wk pr×time interaction term could not be included in the model due to collinearity. For this illness an alternative method of estimating time changes was utilized. The salmonellosis cases were divided into two equal periods of time, those occurring up to 1993 (678 weeks) and those occurring after 1993 (678 weeks). T 2–5 wk pr was significantly associated with excess risk in the earlier time period (relative risk=1·0044, P=0·005) but not in the later period (relative risk=1·0020, P=0·23) providing some evidence that for salmonellosis, T 2–5 wk pr has also diminished in importance over time.
Within these models each temperature variable and its interaction term (e.g. T c,p and T c,p×time) were replaced with interaction terms between the temperature variables and the year of analysis (e.g. T c,p×1974 to T c,p×2006) and the regression re-run. The relative risks of these interaction terms produce an estimate of the impact of temperature upon foodborne illness that particular year. These relative risks were then plotted against year to explore how the impact of temperature has changed over time. The results are presented in Figure 2 for the five foodborne illnesses. In each graph a linear trend line is fitted.
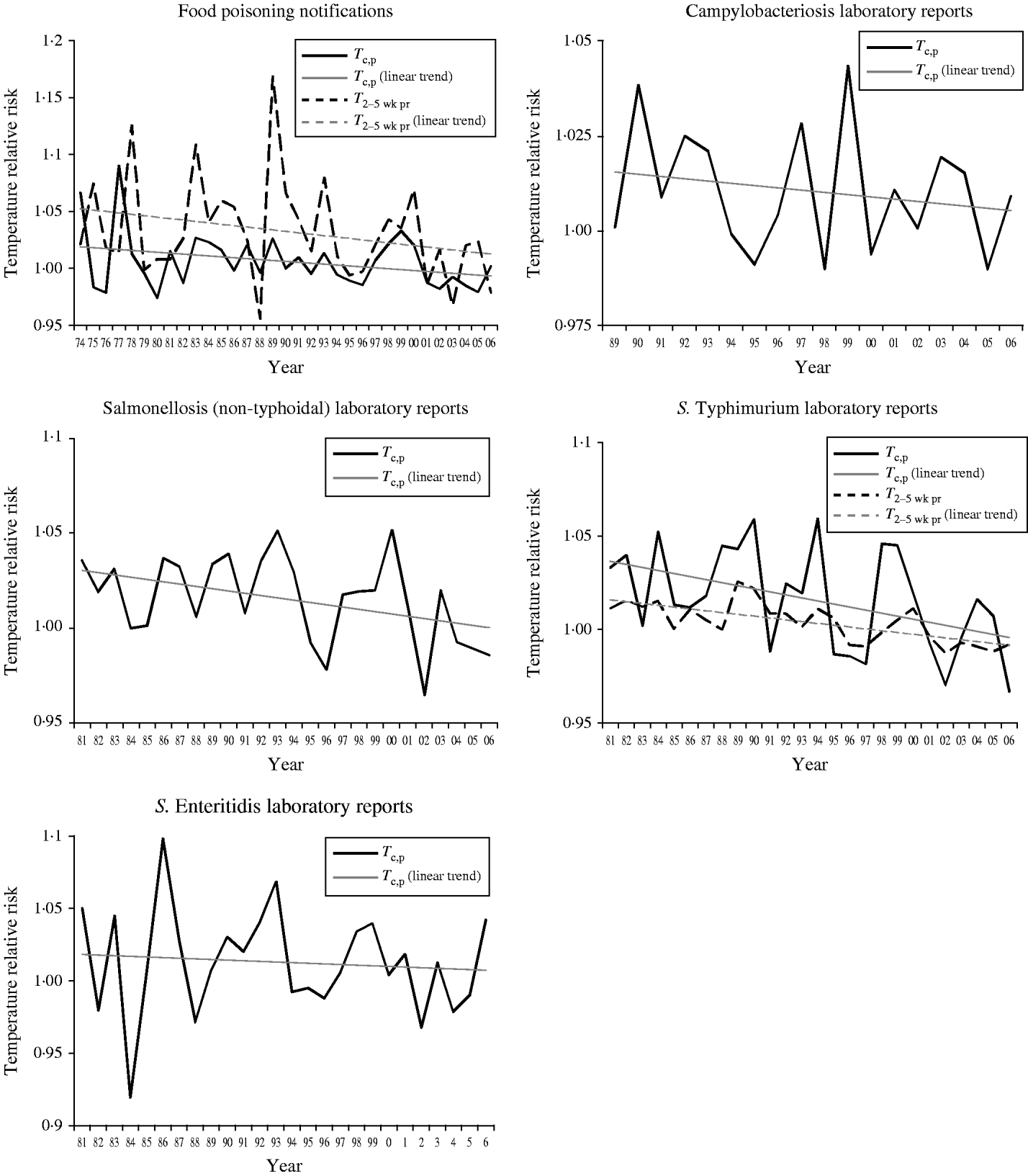
Fig. 2. The variations in the relative risks of temperature over time. T 2–5 wk pr, Temperature 2–5 weeks previously; T c,p, temperature in the current and previous week.
For S. Enteritidis infection and campylobacteriosis the magnitude of the relative risk of T c,p has reduced by half over the years for which data are available. In the case of food poisoning, salmonellosis and S. Typhimurium infection the relative risk becomes 1 suggesting that, using our methodology, short-term changes in T c,p are no longer associated with illness. For food poisoning, the relative risk for T 2–5 wk pr has reduced by half. For S. Typhimurium infection, the relative risk crosses 1 suggesting that short-term temperature changes further from when illness occurred are no longer associated with illness.
Table 1 demonstrates that in all models there was strong evidence of under-reporting in weeks containing a public holiday. There was less consistent evidence of over-reporting in following weeks. Our variable indicating the number of foreign travel-reported cases of illness was significantly associated with excess risk for all illness except campylobacteriosis.
DISCUSSION AND CONCLUSIONS
The paper demonstrates that food poisoning, campylobacteriosis, salmonellosis, S. Enteritidis infection and S. Typhimurium infection were all significantly and positively associated wiith T c,p. Only food poisoning, salmonellosis and S. Typhimurium infections were associated with T 2–5 wk pr. There has also been a significant decrease over time in the impact of T c,p for all illness types except S. Enteritidis infections. The impact of T 2–5 wk pr has also lessened for food poisoning and S. Typhimurium infections.
One limitation of this paper is that it is based on reported foodborne illnesses and these datasets are known to under-represent the true disease burden [Reference Wheeler19]. However, short-term fluctuations in reported incidence should provide a good indication of similar variations in the number of cases in the community. It is also unlikely that short-term variations in the degree of under-reporting (proportion of illnesses in the community of similar severity being reported to national surveillance) would be correlated with temperature [Reference Bentham and Langford6]. Therefore, the results presented are unlikely to be artefactual. Similarly, although reporting completeness may have changed over time, by controlling for long-term trends in the data, this too will not bias our results. There are many statistical methods through which long-term trend and seasonal biases in the data can be controlled for in time-series analysis, and there is no consensus in the literature as to which method is best. The technique chosen in this research, deseasonalizing and detrending the data, will produce conservative estimates of the impact of temperature upon foodborne illness.
All the foodborne illnesses had positive association with T c,p which corroborates previous studies [Reference Kovats4–Reference Bentham and Langford6, Reference Singh9]. These associations might indicate changes in behaviour (e.g. food purchase and barbecues) associated with temperature or reflect the effect of elevated temperatures on bacterial growth in food near to the point of consumption. The latter will not occur for campylobacteriosis, because the organism does not replicate readily outside the gut.
Positive associations between food poisoning notifications and T 2–5 wk pr were also found. This highlights the importance of processes occurring further back in the food chain and hence earlier in time, such as deficiencies in food hygiene during production, processing and distribution. It may also reflect that food poisoning cases are dated to the point of notification and not specimen date. T 2–5 wk pr was not significant for campylobacteriosis which would be expected as the organism does not replicate readily outside the gut. The salmonellosis model showed strong positive associations with T 2–5 wk pr. When the two main Salmonella serovars were considered separately, the effect of T 2–5 wk pr was stronger for S. Typhimurium infection than for S. Enteritidis infection, both in terms of magnitude and significance. This might reflect the levels of contamination in foodstuffs commonly associated with these serovars and their modes of transmission. S. Enteritidis infection is associated with poultry and more commonly eggs. However, most eggs have been free from S. Enteritidis since the early 1990s and any contamination is at low levels. It has also been demonstrated that egg storage at room temperature has no significant effect on Salmonella prevalence [Reference Humphrey20] but will affect the bacterial count in infected eggs. When eggs are pooled or incorporated in dishes in an undercooked or lightly cooked form then the organism is able to proliferate to levels sufficient to cause infection. Such treatment is most likely to occur close to consumption and is corroborated by the observation that around 40% of S. Enteritidis outbreaks are associated with inadequate heat treatment (HPA, unpublished data). S. Typhimurium, is associated with a wider variety of foods. While not limited to cattle, it can be isolated from around 8% of cattle carcases [Reference McEvoy21] and can grow under substandard storage conditions on carcase meat [Reference Kinsella22]. Hence, growth is possible during food production and distribution. Consequently, T 2–5 wk pr is likely to be of greater importance for S. Typhimurium.
The most important finding is that for all foodborne illness, the impact of T c,p has decreased over time. A major factor accounting for this could be the reduction during the 1990s in the incidence of pathogens in food-producing animals. Salmonellas are dominated by two serovars, S. Enteritidis and S. Typhimurium [23]. S. Enteritidis is predominantly phage type 4 [Reference Gillespie24] and many poultry producers have joined a voluntary agreement to vaccinate their flocks against S. Enteritidis [25]. This started for broiler chickens in 1994 and commercial layer flocks in 1997. By 1999 it was estimated that 85% of layers in production had been vaccinated [25] and by 2003 a large reduction in S. Enteritidis contamination of raw eggs was also reported [26]. During the mid to late 1990s S. Typhimurium was dominated by definitive phage type (DT)104. This is predominantly, but not totally, associated with cattle and the emergence of a subtype resistant to multiple antibiotics in the early 1990s prompted initiatives across the industry to counteract the increase such as limiting the use of antimicrobial growth promoters and improved carcase disinfection at abattoirs [Reference Cutter and Rivera-Betancourt27]. These initiatives coincided with a reduction in S. Typhimurium infections from the late 1990s onwards (Fig. 1). Finally, since the late 1990s biosecurity improvements at poultry farms have been associated with lower Campylobacter levels in chicken.
The studies presented above provide strong evidence that there has been a reduction in the pathogen load of many foods. In the case of salmonellas, which are thermophilic and under the correct conditions will reproduce at higher temperatures, this means that if any contamination remained within the product it would have the potential to be at lower levels, suggesting that it would take a greater temperature event to result in an infective dose. This could explain why temperature appears to play a lesser role for all salmonellas than it did in the past. Because campylobacters do not readily replicate outside the gut, this explanation does not apply to them. However, for both salmonellas and campylobacters, if foods infected with these organisms are preferentially eaten during periods of elevated temperature then an association between illness and temperature will be observed. If, as argued, an increasing proportion of these foods have become free from salmonellas and campylobacters, this will diminish the apparent role of temperature.
The reduction in the risk of temperature was constant over time and in the model interactions between temperature and polynomials of time were not significant. This indicates that, in addition to the reduction in the pathogen load of major food groups occurring in the 1990s, other factors are also likely to have played a role in this reduction. Another possibility is improvements in food hygiene which may prevent cross contamination, reduce bacterial reproduction and kill foodborne organisms. There is some evidence that these have occurred. Improved disease surveillance, microbial diagnostic methods and the increasing numbers of reported foodborne illnesses since the 1970s has increased governmental awareness of this issue. This has led to initiatives to improve food hygiene at all stages of the food production process. For example the 1970 Food Hygiene Regulations were strengthened twice in the 1990s. In 1995 the Food Safety (General Food Hygiene) Regulations came into force introducing Hazard Analysis and Critical Control Points methodologies to food safety [Reference Adams28]. At the household level, improvements in food hygiene may be evidenced from increases in the numbers of households with refrigerators which only reached saturation in the mid 1980s [29]. During the past 30 years there have also been several highly publicized foodborne outbreaks (e.g. the Wakefield outbreak of S. Typhimurium in 1985, which led to 19 deaths [30]). Combined with a number of public campaigns (e.g. national food safety weeks) these may have led to increased awareness of correct food hygiene behaviour in the home.
This paper also presents evidence that for food poisoning notifications and S. Typhimurium infections T 2–5 wk pr has become less important. This again points to improvements in food pathogen load and food hygiene, but this time occurring further in time from the point of consumption and so probably in the food production process.
These results provide new evidence on the potential impact of climate change upon human health. Previous studies have inspected the current impact of temperature upon foodborne illness and extrapolated it into the future [Reference Bentham and Langford6, Reference McMichael, Woodruff and Hales11, Reference Ebi12]. The results presented in this paper indicate that temperature still plays an important role in foodborne infections, but in England and Wales, the impact of temperature upon foodborne illness is decreasing over time. Consequently, the papers cited above are likely to exaggerate the potential impact of climate change. Finally, this paper demonstrates how England and Wales are successfully adapting to the threat of increased foodborne illnesses posed by climate change through reducing the pathogen levels in major food groups and improving food hygiene at the domestic and institutional level. Such approaches could be adopted elsewhere.
DECLARATION OF INTEREST
I. A. Gillespie, G. L. Nichols, C. Lane, G. K. Adak and E. J. Threlfall are employees of the Health Protection Agency.





