Introduction
Since December 2019, a cluster of unexplained pneumonia cases has been reported in Wuhan, Hubei Province. This was subsequently identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this was named, coronavirus disease 2019 (COVID-19), by the World Health Organization (WHO) [1]. As of 13 July 2020, 12 552 765 cases have been infected by SARS-CoV-2, and 561 617 patients have died worldwide. Furthermore, 216 countries have been affected, posing a major threat to human public health [2]. To date, a specific treatment for SARS-CoV-2 has not been recommended, except for meticulous supportive care. Therefore, it is crucial to identify risk factors that are associated with the poor prognosis of patients with COVID-19. Some studies suggested that older age, comorbidities (hypertension and cardiovascular diseases), sequential organ failure assessment scores and some laboratory indices, such as neutrophil, D-dimer, lymphocyte, interleukin (IL)-6 and C-reactive protein, are associated with the development of poor prognosis [Reference Zhou3–Reference Wang8]. However, some of these risk factors are cumbersome and costly. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are novel biomarkers that provide important information about the systemic inflammation status, and these are easily available from routine laboratory studies. The elevated NLR and PLR are significantly associated with the mortality of patients with infectious diseases [Reference Curbelo9–Reference Zhao12]. Therefore, recent studies have suggested that NLR is an effective predictor for the mortality of patients with COVID-19 [Reference Qin4, Reference Liu13, Reference Lagunas-Rangel14]. The dynamic increase of PLR during the hospitalisation might suggest the severity and prognosis of the disease [Reference Qu15]. However, to date, no studies have simultaneously explored the values of NLR and PLR in predicting the mortality in COVID-19. In the current study, the investigators aimed to determine whether the PLR can serve as a valuable predictor of in-hospital mortality, and the value of NLR for predicting the all-cause mortality in patients with COVID-19.
Methods
Study design and participants
The current retrospective cohort study included 151 patients with COVID-19 in Wuhan No. 1 Hospital from 13 February 2020 to 14 March 2020. COVID-19 was diagnosed according to the Seventh Edition of the Interim Guidance of the National Health Commission of the People's Republic of China [16]. The patients were excluded from further analyses when they had an active condition at the time of COVID-19, which could significantly influence the blood cell count, including chronic obstructive pulmonary disease (COPD). Furthermore, patients that did not have a record of their blood cell count were also excluded (Fig. 1). The epidemiology, demography, clinical manifestations, laboratory examination and outcome were extracted from the electronic medical records by the Harbin Aid Hubei Medical Team. Most of the clinical data were collected from the first day of admission, unless otherwise noted in the current study. For severe pneumonia (meeting any of the following): (1) dyspnoea, respiratory rate of ≥30 breaths/min; (2) peripheral oxygen saturation ≤93% at rest and (3) oxygen partial pressure/oxygen uptake fraction of ≤300 mmHg (1 mmHg = 0.133 kPa). For non-severe pneumonia: the above criteria were not met. The definition of non-effective antibiotic treatment (meeting any of the following): (1) no decrease in temperature after 48–72 h of antibiotic therapy and (2) no improvement in symptoms after 48–72 h of antibiotic therapy. All patients included in the current study had a definite outcome (death or discharge). The current study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (KY2020-011).
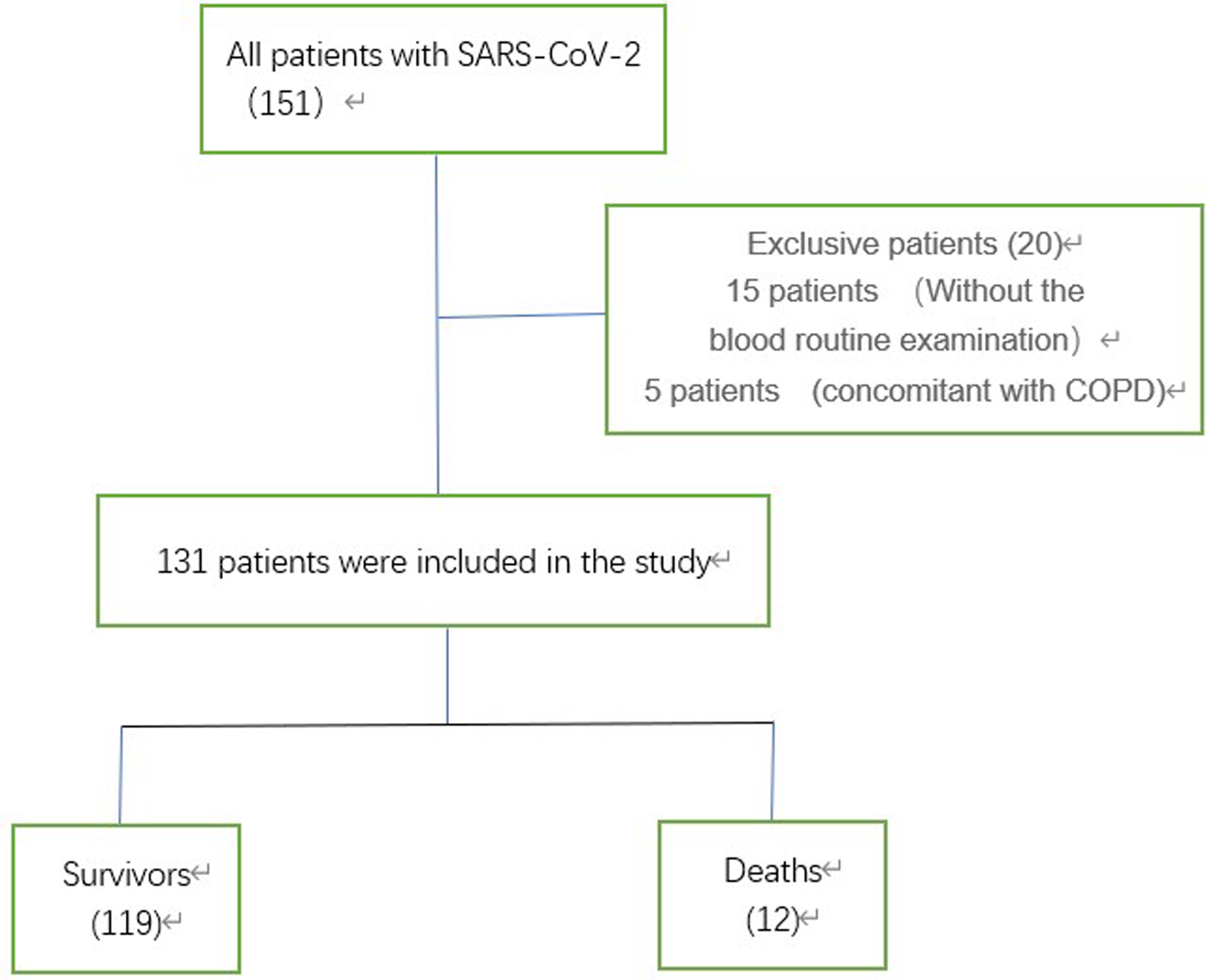
Fig. 1. The study flow chart. COPD, chronic obstructive pulmonary disease; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Statistical analysis
SPSS Statistics 25 (IBM SPSS) was used for the statistical analyses. The categorical variables were described as the number/total number (%), and continuous variables were described using the mean, median and interquartile range (IQR) values. The Kolmogorov–Smirnov test was used to evaluate the data for the normality of the distribution. The means for continuous variables were compared according to the independent group t-tests, when the data were normally distributed. Otherwise, the Mann–Whitney test was used. The categorical data were compared by χ 2 test or Fisher's exact test. The receiver operating characteristic (ROC) curve was used to state the sensitivity and specificity of NLR and PLR for all-cause mortality and disease severity. The Youden index was calculated to determine the optimal cut-off values. In order to explore the risk factors associated with in-hospital mortality, the logistic regression model was used. Bilateral test (the test level α = 0.05) was used, and P < 0.05 was considered statistically significant.
Results
Demographic characteristics
A total of 131 patients with COVID-19 were included in the current study. The demographic characteristics of these patients are shown in Table 1. Among the 131 patients with a median age of 64 years old (IQR: 56–71), 12 (9.2%) patients died. There were no significant differences in gender and comorbidities between these two groups. In the non-survivor group, more patients had severe pneumonia (33.3% vs. 13.4%, P = 0.087). Approximately 87% of patients received varying degrees of antibiotic therapy during their hospitalisation, and more non-survivors were non-effective to antibiotic treatment (70% vs. 15.4%, P < 0.001). Compared to the survivors, the non-survivors were older (median age 80 (IQR: 70–85) vs. 64 (IQR: 56–69), P < 0.01), and were more likely to present with initial symptoms of dyspnoea (12 (100%) vs. 15 (12.6%), P < 0.01). However, more survivors presented with initial symptoms of cough (85 (71.4%) vs. 4 (33.3%), P = 0.018), when compared with the non-survivors.
Table 1. Comparison of the demographic characteristics of survivors and non-survivors with COVID-19 pneumonia
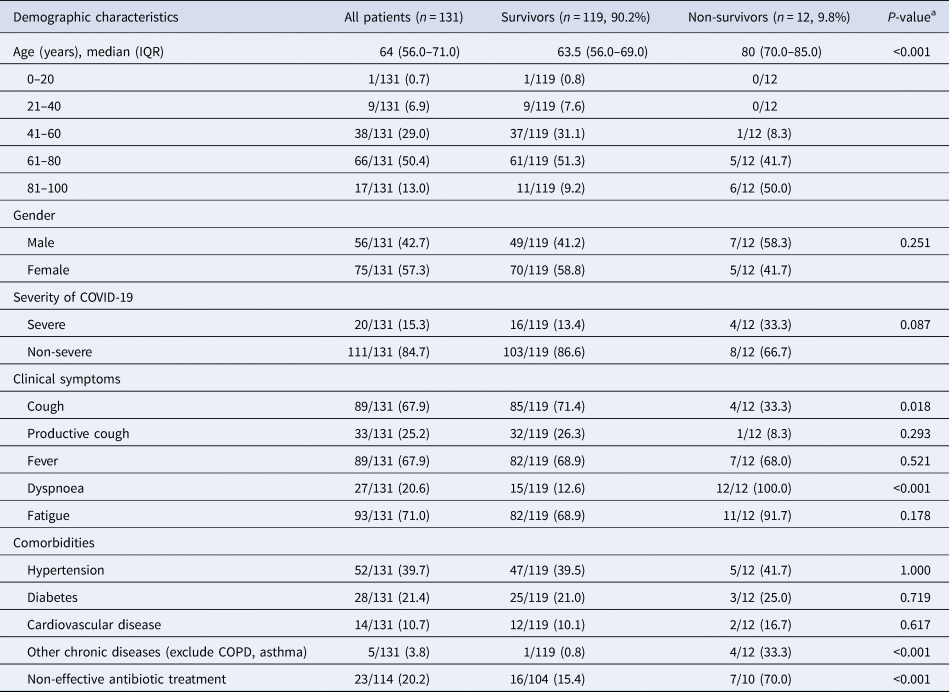
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; IQR, interquartile range.
Data are expressed as median (IQR) or n/N (%).
a Mann–Whitney U test, χ 2 test or Fisher's exact test.
Initial laboratory indices
The initial laboratory indicators of surviving and dead patients are presented in Table 2. The leucocytes (6.03 × 109/l vs. 11.66 × 109/l, P < 0.001) and neutrophils (3.73 × 109/l vs. 10.07 × 109/l, P < 0.001) were significantly higher in non-survivors. The lymphocyte counts (1.53 ± 0.61 × 109/l vs. 0.74 ± 0.38 × 109/l, P < 0.001) and platelet counts (240 × 109/l vs. 158.27 × 109/l, P = 0.007) were significantly lower in non-survivors. The value of aspartate aminotransferase (AST, 23 U/l vs. 48 U/l, P < 0.01) was higher in non-survivors. At the same time, the albumin level in the non-survivor group was lower (33.81 g/l vs. 29.16 g/l, P = 0.008), while the globulin level was higher (27.6 ± 3.14 g/l vs. 33.27 ± 6.97 g/l, P = 0.032). These results revealed that the albumin/globulin ratio for non-survivors was lower (A/G, 1.27 ± 0.25 vs. 0.90 ± 0.17, P < 0.001). Furthermore, the levels of lactate dehydrogenase (LDH, 200.5 U/l vs. 721.0 U/l, P < 0.01) and blood urea nitrogen (BUN, 4.10 mmol/l vs. 9.70 mmol/l, P < 0.01) were higher in non-survivors. The results revealed that the values of alanine aminotransferase (24 U/l vs. 35 U/l, P < 0.01), creatine kinase (51.0 U/l vs. 107.0 U/l, P = 0.017) and serum creatinine (61.5 μmol/l vs. 95.0 μmol/l, P = 0.04) were all within the normal range in these two groups. There were no statistical differences in the other laboratory indices between these two groups.
Table 2. Comparison of the initial clinical laboratory data between survivors and non-survivors with COVID-19
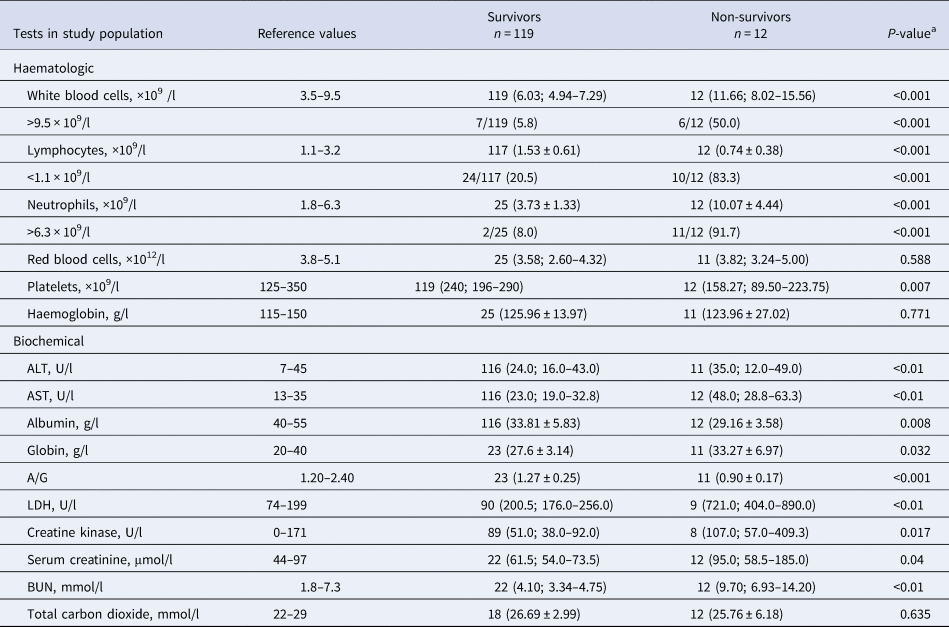
ALT, alanine aminotransferase; AST, aspartate aminotransferase; A/G, albumin/globulin ration; BUN, blood urea nitrogen; COVID-19, coronavirus disease 2019; LDH, lactate dehydrogenase.
Data are shown as n (median, IQR), n (mean ± standard deviation) or n/N (%).
a Student's t-test or Mann–Whitney U test was used for continuous data, χ 2 test or Fisher's exact test for categorical variables.
As shown in Table 3, NLR was significantly elevated in non-survivors, when compared to survivors (13.87 (7.50–24.82) vs. 1.95 (1.43–2.58), P < 0.001). However, there were no significant differences in PLR for non-survivors, when compared to survivors (P = 0.251). In addition, it was found that NLR was higher in the severe group (6.88 (3.54–11.18) vs. 2.21 (1.51–9.85), P = 0.065) after grouping the patients according to their severity, and PLR had similar results (195.97 (157.75–246.05) vs. 165.89 (112.90–227.96)), but the difference was not significant (P = 0.104).
Table 3. The value of NLR and PLR for COVID-19

COVID-19, coronavirus disease 2019; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
* The P-value of NLR is <0.001 and 0.251 for PLR.
# The P-value of NLR is 0.065 and 0.104 for PLR.
Univariate analysis for risk factors
As shown in Table 4, the univariate analysis revealed that age (odds ratio (OR) 1.116, 95% confidence interval (CI) 1.050–1.187, P < 0.01), white blood cell count (OR 1.419, 95% CI 1.117–1.711, P < 0.001), lymphocytes (OR 0.028, 95% CI 0.004–0.400, P < 0.001), neutrophils (OR 2.265, 95% CI 1.284–3.997, P < 0.001), AST (OR 1.034, 95% CI 1.012–1.057, P = 0.002), albumin (OR 0.855, 95% CI 0.756–0.966, P = 0.012), A/G ratio (OR 0.000, 95% CI 0.000–0.085, P = 0.005), LDH (OR 1.008, 95% CI 1.003–1.012, P < 0.001), creatine kinase (OR 1.004, 95% CI 1.001–1.007, P = 0.020), serum creatinine (OR 1.025, 95% CI 1.001–1.048, P = 0.037) and BUN (OR 1.329, 95% CI 1.050–1.682; P = 0.018) were significantly correlated with death induced by COVID-19.
Table 4. The univariate and multivariate analysis for risk factors associated with death in patients with COVID-19
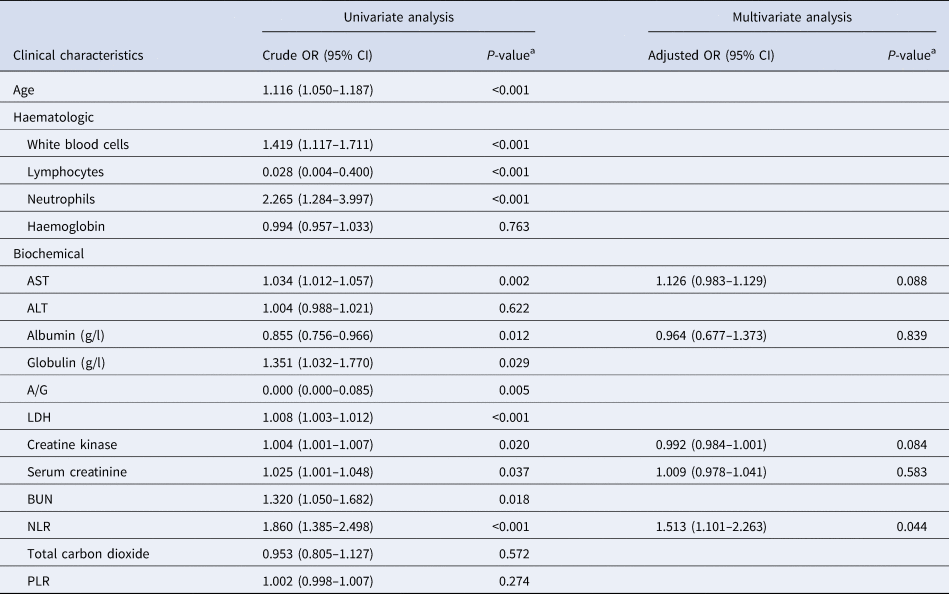
A/G, albumin/globulin ration; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
a Logistic regression analysis.
Multivariate analysis for risk factors
Considering the results of the univariate analysis and the problem of sample size, five serological indices (creatine kinase, albumin, AST, serum creatinine and NLR) were finally included in the multivariate analysis to determine the close relationship between NLR and death (Table 4). After excluding the influences of the other four factors, NLR was still closely correlated with death (adjusted OR 1.513, 95% CI 1.001–2.263, P = 0.044), suggesting that NLR may be a valuable biomarker in response to mortality in COVID-19.
ROC curve analysis
Based on the ROC curve analysis (Fig. 2), the NLR of 3.338 was associated with all-cause mortality, with a sensitivity of 100.0% and a specificity of 84.0% (area under the curve (AUC): 0.963, 95% CI 0.911–1.000; P < 0.001). In view of the small number of deaths (n = 12) in the current study, the NLR of 2.306 might have potential values in helping clinicians to identify patients with severe COVID-19, with a sensitivity of 100.0% and a specificity of 56.7% (AUC: 0.729, 95% CI 0.563–0.892; P = 0.063). However, the PLR has no observed value for distinguishing the severity (AUC: 0.614, 95% CI 0.489–0.704, P = 0.104) and predicting the death of patients with COVID-19 (AUC: 0.601, 95% CI 0.414–0.788, P = 0.251).
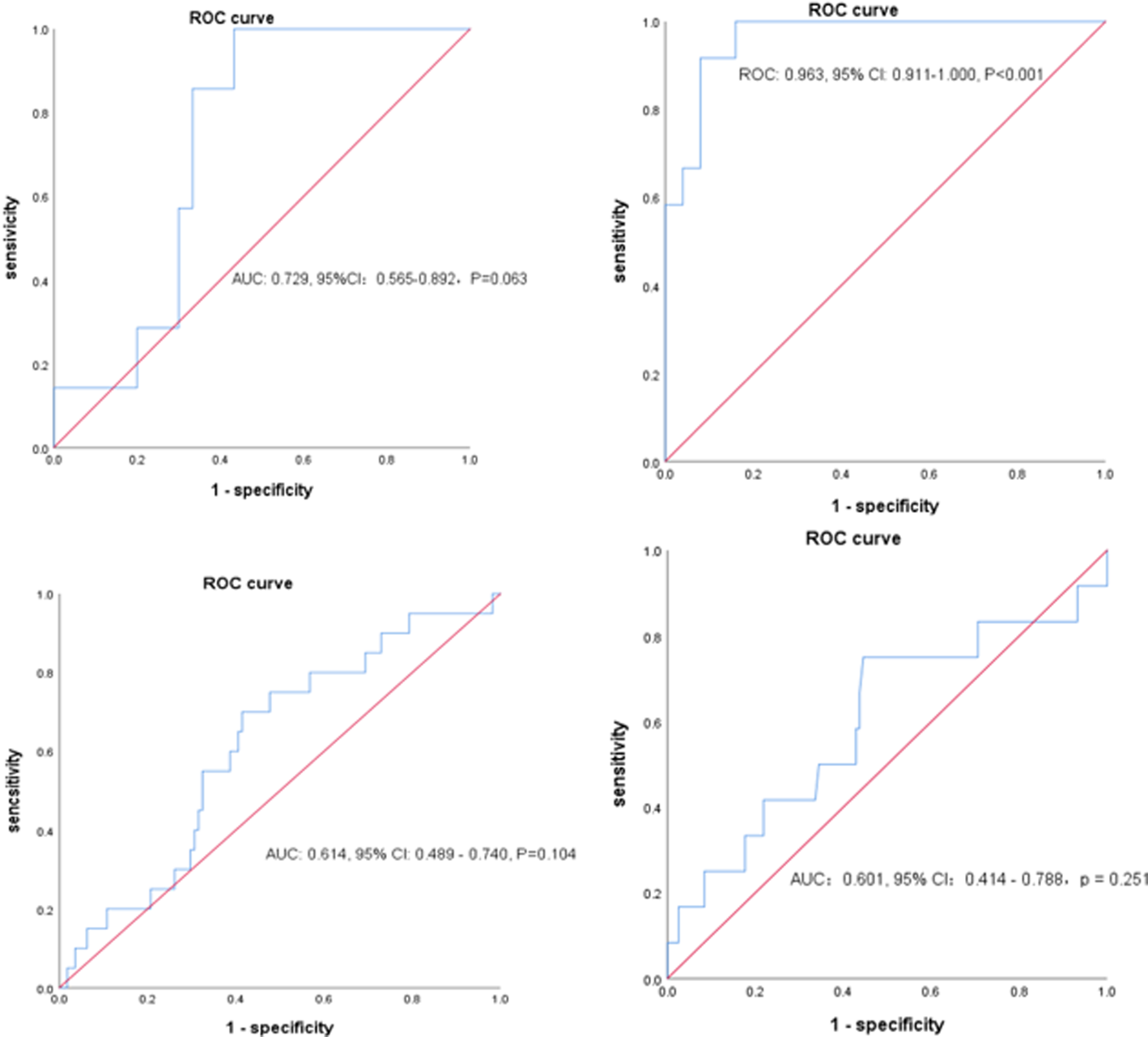
Fig. 2. The patient profiles demonstrate the sensitivity and specificity of the following: (A) NLR in predicting severity, (B) NLR in predicting death, (C) PLR in predicting severity and (D) PLR in predicting severity. AUC, area under the curve; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Discussion
The current study suggests that the elevated NLR is associated with all-cause mortality in patients with COVID-19, while PLR was not associated with this. The NLR was significantly higher in non-survivors, when compared to survivors, which is consistent with the reports of other studies [Reference Qin4, Reference Lagunas-Rangel14]. The logistic regression analysis revealed that the NLR is associated with the mortality of COVID-19 (crude OR 1.860, 95% CI 1.385–2.498). After adjusting the other confounding factors, the NLR remained as a risk factor for COVID-19 (adjusted OR 1.513, 95% CI 1.101–2.263). It was also demonstrated that the NLR of 3.328 has a good predictive value of all-cause mortality in patients with COVID-19, with a sensitivity of 100.0% and a specificity of 84.0%. In the current study, the elevated NLR may serve as a diagnostic indicator for severe COVID-19, and this has been shown in other studies [Reference Qin4, Reference Fei17]. In addition, it was found that there are many biochemical indicators closely correlated with death, such as AST, creatine kinase and serum creatinine, which are consistent with the reports of other studies [Reference Zhou3, Reference Fu6, Reference Wang8, Reference Guo18, Reference Wu19]. These risk factors suggest that these dead patients might have had multiple organ damage at the beginning of the hospitalisation. Similar to other studies [Reference Zhou3, Reference Wang8, Reference Wu19], age was also closely correlated with death in the current study. Comorbidities (hypertension, diabetes and cardiovascular disease) that have been shown to be associated with death did not differ between survivors and non-survivors [Reference Zhou3, Reference Wang8, Reference Alger20], which may be due to the population heterogeneity.
As it is known, the human immune system plays a major role in putting out viral infections. The NLR reflects that the high systemic inflammatory response is associated with the poor prognosis of infectious diseases [Reference Curbelo9–Reference Zhao12]. Several studies have manifested that severe cases (including dead patients) of COVID-19 were more likely to have higher neutrophil counts and lower lymphocyte counts, when compared with non-severe cases. Thus, the elevated NLR tends to predict the severity of COVID-19 [Reference Qin4]. Through a retrospective analysis of 452 patients, Qin et al. [Reference Qin4] reported that severe cases are likely to have higher NLRs caused by the higher neutrophil counts, but these cases would have lower lymphocyte counts, when compared to non-severe patients, indicating that the surveillance of NLRs might be helpful for the early screening of the critical illness of COVID-19. Furthermore, both helper T cells and suppressor T cells were below the normal levels, and the decline in helper T cells was more pronounced in severe cases that have been proven to be a key point in the weakening or suppressing overactive immune responses of SARS [Reference Li21]. Diao et al. [Reference Diao22] reported that T cells significantly decreased in patients in the intensive care unit (ICU), when compared to patients who were not in the ICU. Furthermore, the total T cells, and CD4+ and CD8+ T cells in severe and critical patients were significantly lower, when compared to the mild/moderate diseases in patients who were not in the ICU [Reference Diao22]. In a report from China, minimally invasive autopsies were performed on three patients [Reference Yao23]. In addition to the known severe pulmonary lesions, there was a marked disturbance in the lymphatic haematopoietic system. Splenic lymphocytosis, cellular degeneration and necrosis presented in the lymph nodes with reduced lymphocytes and focal necrosis. As a retrospective study, due to the limitations of the isolation ward at the beginning of the epidemic and the urgency of containing the COVID-19 epidemic, the study did not document some data in detail, such as the dynamics of the laboratory indicators. Hence, the investigators referred to the data obtained by other researchers. In the study conducted by Tan et al. [Reference Tan24], lymphopaenia was found to be a predictor of prognosis in patients with COVID-19 pneumonia. In their time-to-lymphocyte percentage (LYM%) curve model, at 10–12 days after symptom onset, patients with LYM% <20% were initially classified as severe. At 17–19 days after onset, patients with LYM% <20% were still at risk, and required monitoring. Patients with LYM% <5% were critical, had a high mortality rate, and required intensive care. The NLR was higher for identifying patients with severe pneumonia, when compared to that for identifying those who have died, suggesting that a higher NLR might predict the progression during hospitalisation [Reference Long25]. In the study conducted by Ding et al. [Reference Ding26], the NLR was significantly higher in severe patients, when compared to non-severe patients, at all time points after hospital admission, and the NLR was positively correlated with hospitalisation time from day 5 after admission. These findings might be explained by the following reasons: (1) angiotensin-converting enzyme 2 (ACE2) has been considered as the main receptor for SARS-CoV-2, which may be expressed in lymphocytes, cause the SARS-CoV-2 to directly infect these cells, and ultimately bring about lymphopaenia [Reference Hoffmann27], leaving the body vulnerable to bacterial invasion and inducing an increase in neutrophils [Reference Qin4]. (2) The cytokine storm has been considered as an important contributor to respiratory viral infections [Reference Huang28], while cytokines, such as IL-10, IL-6 and tumour necrosis factor-α, might activate the neutrophils and damage the lymphocytes [Reference Long25, Reference Zhang29]. Thus, the NLR may reflect the level of severity of COVID-19. It was also found that the antibiotic inefficiency was higher in the non-survival group, which might also suggest that inflammatory storms are involved in the progression of COVID-19. However, due to the number of deaths (n = 12), further studies, particularly prospective studies, are needed to ascertain the value of the NLR in mortality.
In addition, the current study explored the association between PLR and all-cause mortality in COVID-19. Platelets are important immune cells in the human body, which are produced by mature megakaryocytes in the bone marrow. This plays an important role in blood coagulation, angiogenesis, immunity and inflammation [Reference Nicolai and Massberg30]. The mechanism of thrombocytopaenia may be the joint action of many factors. There are many similarities between the outbreak of COVID-19 and the outbreak of SARS in 2003. Previous studies on SARS suggested that the reasons might be, as follows: (1) the viral infection and mechanical ventilation led to endothelial injury, platelet aggregation, pulmonary thrombogenesis [Reference Yang31] and megakaryocyte reduction, and as a result, the platelet production decreased and the consumption increased; (2) the coronavirus directly invades haematopoietic cells or bone marrow stromal cells, resulting in haematopoiesis inhibition [Reference Eickmann32]. Indeed, there are marked differences in the physical and chemical properties between SARS and COVID-19. However, it remains to be determined whether this phenomenon can be explained by the same mechanism. As a new inflammatory index, the PLR can reflect the infection and factor aggregation [Reference Gasparyan33], which is more valuable, when compared to the simple platelet or lymphocyte count. By comparing the dynamic changes of the PLR during hospitalisation, Qu R et al. [Reference Qu15] reported that the increase in PLR was correlated with the poor prognosis on COVID-19. Unfortunately, due to the low sample size and mortality rates, no value was identified in the current study.
Clinical implications and strengths
First, patients with COPD were excluded, which reduced the impact of long-term inhaled glucocorticoids and airway inflammation on the outcomes. Second, COVID-19 imposes a significant global medical and economic burden. Compared with factors, such as IL-6, which respond to inflammatory markers, NLRs are simple, fast and inexpensive to obtain directly from the blood, and this can help clinicians identify the serious illness and prognosis of COVID-19, thereby allowing for the aggressive adjustment of treatment plans to reduce patient death.
Limitations
Frist, due to the retrospective study design and limited sample size, the real value of the PLR might be underestimated in predicting the in-hospital death. Hence, further studies are needed to ascertain the real value of the NLR and PLR in predicting the mortality for COVID-19. Second, the investigators did not compare the NLR and PLR changes in the progress of COVID-19 with the values at baseline. This may lead to the misunderstanding of the predictions of biomarkers in all-cause mortality in COVID-19 [Reference Qu15].
Conclusion
NLR is a simple biomarker that reflects the presence of systemic inflammation, and is associated with all-cause mortality in COVID-19. The elevation of NLR was a useful biomarker to predict the mortality in COVID-19. Further studies are needed to ascertain the dynamic values of the NLR in predicting all-cause mortality in COVID-19, and explore more useful markers to timely detect critical patients.
Acknowledgements
We acknowledge the First Hospital of Wuhan for providing the medical records.
Author contributions
D.J.S., L.L., Q.W., S.Z., J.W.W. and X.N.Z. performed the data collection. X.C.L., X.W. and Y.S. prepared the first draft of the manuscript, validated the data collection, refined the research idea, performed the data analysis and edited the manuscript. H.C. and Y.P.L. developed the research idea, refined the research idea, validated the data collection and edited the manuscript. H.C. and Y.P.L. are the guarantors of the manuscript.
Conflict of interest
None.
Ethical standards
The ethics committee of the Second Affiliated Hospital of Harbin Medical University approved this study, and granted a waiver of informed consent from the study participants (KY2020-011), which was authorised by the Ethics Committee of Wuhan No. 1 Hospital.
Data available statement
The data that support the findings of this study are available from the corresponding author, HC, upon reasonable request.









