INTRODUCTION
Rabbit haemorrhagic disease virus (RHDV) was introduced into Australia in 1995 and largely fulfilled expectations that it would provide major economic and ecological benefits by reducing the abundance of introduced wild rabbits Oryctolagus cuniculus [Reference Fenner and Fantini1]. Rabbit numbers fell by about 95% in arid areas [Reference Bowen and Read2, Reference Mutze, Cooke and Alexander3], but lesser population declines (68%) or even increases were seen in some humid regions [Reference Saunders4]. In a high rainfall site at Cattai National Park, New South Wales, RHDV was unable to establish despite several deliberate attempts at introduction [Reference Richardson5]. Analysis of data from across Australia showed that disease impact declined along a hot-dry to cool-wet gradient [Reference Henzell, Cunningham and Neave6] and generally reduced Australia's wild rabbit population by about 60%.
Nonetheless, since 2003, rabbits have been noticeably increasing once more, especially in areas of intermediate to low rainfall in central and northwestern Victoria [Reference McPhee7, Reference Sandell8]. An increase of this kind was anticipated given that rabbit populations had regained some of their former abundance 10–15 years after the myxoma virus was introduced into Australia to control rabbits in 1950 [Reference Fenner and Ratcliffe9]. In the case of the earlier myxoma virus release, rabbits not only began to show genetic resistance to infection [Reference Fenner and Fantini1, Reference Best and Kerr10], but the myxoma virus also attenuated into a range of less virulent field strains. Since the mid-1960s field strains of myxoma virus have apparently maintained their relative virulence killing between 40% and 60% of infected rabbits as the virus has co-evolved to keep pace with the rabbits' ever increasing resistance [10, 11, B. D. Cooke, unpublished observations].
However, it cannot be assumed that changes in susceptibility to rabbit haemorrhagic disease (RHD) should follow the same patterns as observed with myxomatosis. Major differences between the two viruses must be taken into account. The most important of these is that upon release, the myxoma virus was a completely new virus affecting O. cuniculus, having been transferred from Sylvilagus braziliensis, whereas, there is growing evidence that RHDV is essentially a new pathogenic variant of a genus of generally non-pathogenic rabbit-specific lagoviruses that had long circulated in both wild and domestic rabbits in Europe [Reference Capucci, Scicluna and Lavazza12–Reference Kerr, Kitchen and Holmes14]. This raises the possibility that RHDV is pre-adapted to rabbits and greater resilience to RHDV may not be based on an amelioration of acute generalized disease at a cellular level as seen in myxomatosis [Reference Best and Kerr10].
In this study we sought to establish whether rabbits were developing resistance to RHD. We used field-caught rabbits in challenge experiments, as had previously been done for myxoma virus, but we considered, as well, the nature of the underlying mechanisms of resistance. We aimed to provide broad background information and research directions for newer molecular techniques to more precisely identify the resistance mechanisms and genes involved. As we have no baseline data from the period when RHDV was introduced we began with the null-hypotheses that (a) there should be no significant differences between infection rates caused by the virus in wild rabbits and unselected domestic rabbits and (b) that there should be no differences in infection rates between rabbits from different sites within Australia.
Previous studies have demonstrated that the impact of RHDV on rabbit populations declines in cooler, wetter areas [Reference Henzell, Cunningham and Neave6] and the recent isolation of a non-pathogenic calicivirus, RCV-A1 [Reference Strive, Wright and Robinson15] provides an explanation as to why rabbits in wetter areas may be less affected by RHD [Reference Henzell, Cunningham and Neave6, Reference Cooke16–Reference Bruce and Twigg18]. RCV-A1 is restricted to relatively high rainfall regions of southeastern Australia [Reference Jahnke19], and antibodies raised against it provide temporary protection against acute RHDV infection [Reference Strive20]. On this basis we further postulate that, if genetic resistance to RHDV was evident in any of the wild rabbits sampled from different localities, then we might expect a gradient in resistance with rabbits from higher rainfall areas (>400 mm rainfall annually) having least resistance. Such an observation could potentially provide a consistent, underlying pattern to the data and subtly reinforce the idea of increasing genetic resistance to RHDV.
METHODS
Rabbit collection and management
Rabbits were collected from 12 sites (see Table 1 for details) in both arid inland Australia and in cooler, more humid coastal regions of southeastern Australia. Rabbits were caught using wire cage traps, baited with carrots or oats and a 1 ml blood sample was collected from each rabbit to test for previous exposure to RHDV. To avoid the problem that rabbits collected may have previously been exposed to RHDV, and that samples were biased towards those that were resistant to infection, we collected rabbits at times of the year when RHDV was least active (winter and early spring). We also preferentially collected rabbit kittens aged much less than 12 weeks to further reduce this problem. When RHDV was noted in an area where trapping had commenced, we moved traps to alternative sites where there was no evidence that RHDV was actively spreading.
Table 1. Site description, infection rate, mortality rate and survival times for each population of rabbits orally challenged with 60 ID50 doses of rabbit haemorrhagic disease virus
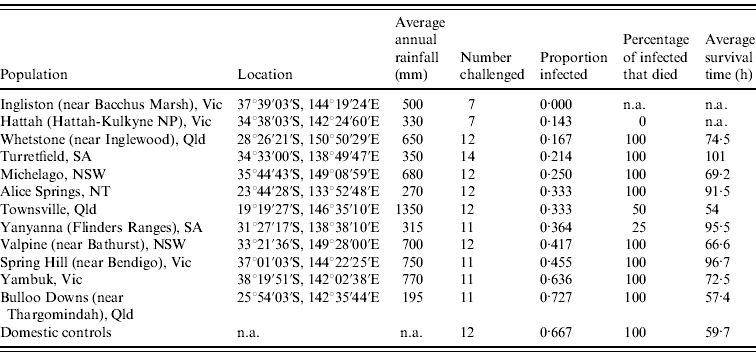
n.a., Not available.
Sera separated from the blood samples were immediately sent to the Natural Resources Management Biosecurity Unit, Biosecurity SA, Adelaide for analysis using a series of ELISA tests: competition ELISA, and specific ELISAs to obtain IgG, IgM and IgA titres [Reference Capucci21]. This enabled classification of rabbits as seronegative to RHD, seropositive with antibodies of maternal origin, seropositive with antibodies apparently raised against RCV-A1 or seropositive survivors of RHD [Reference Cooke22].
On return of the ELISA results, seropositive rabbits were euthanized and seronegative rabbits and those judged to have temporary maternal antibodies were taken to the Robert Wicks Pest Animal Research Centre, Inglewood, Queensland (Biosecurity Queensland, Department of Employment, Economic Development and Innovation) where they were held in quarantine for 10 days to ensure that none were incubating RHD, RCV-A1 or myxomatosis. When all had become fully susceptible as young adults (age 12 weeks, body weight ⩾900 g) fresh serum samples from each rabbit were assayed to confirm that they remained seronegative. Rabbits with low but equivocal traces of antibodies were considered unreliable for experimental purposes and were withdrawn from experiments (a total of 15 rabbits, five each from Ingliston and Hattah, and one each from Yanyanna, Spring Hill, Yambuk, Bulloo Downs and Alice Springs, were withdrawn from the 147 collected); this reduced samples from some sites to below the ten rabbits generally sought for testing.
For experimental challenge, rabbits were individually housed in plastic boxes (610×410×400 mm) with insect-proof gauze lids and wire mesh floors over absorbent litter. These were held in a climate-controlled room [22±1°C, 50% relative humidity, 12-h light/dark cycle (lights on: 06:00 hours)]. Water and food (commercial rabbit pellets and fresh carrot) were available ad libitum.
It was not possible to test all rabbits simultaneously in one large experiment because of the large number of rabbits used and difficulties in obtaining seronegative rabbits from some sites. However, the use of an animal house where trial conditions could be tightly controlled reduced environmental variation that might otherwise confound experimental results.
Domestic rabbits, also aged >12 weeks, obtained from a commercial supplier (Mr I. Handebo, ‘Deeford’, Australia) were used to establish an appropriate challenge dose and were also included as experimental controls when wild rabbits were challenged. All were seronegative on initial testing and because the parental ‘Crusader’ rabbits had previously been sourced from CSIRO (FD McMaster Laboratory, Armidale, NSW) they could be confirmed as having had no prior exposure to RHDV (Dr Sandra Eady, CSIRO, personal communication).
Animal ethics approval
Approval was received from: Queensland Pest Animal Ethics Committee approval PAEC060601; South Australia Wildlife Ethics Committee approval WEC45/2007; PIRSA Animal Ethics Committee approval AEC09/03; and Victorian Animal Ethics Committee approval 062793.
Virus challenge
Most wild rabbits can be infected with a large dose of commercially available Czech strain RHDV (0·5 ml 1500 ID50) but to detect evidence of resistance in different populations it was important to use a lesser challenge dose rather than an overwhelming dose. The methods used to select an appropriate dose will appear in a forthcoming paper, but briefly, we used groups (n=5) of the same domestic rabbits used as experimental controls and challenged them with 1:10, 1:20, 1:33, 1:50, 1:100 and 1:300 dilutions as 0·5 ml of solution. We finally selected a challenge dose, equivalent to 60 ID50 Czech RHDV in 0·5 ml (a 1:25 dilution of the stock solution), which infected two thirds of unselected domestic rabbits. We anticipated that some wild rabbits could have developed high levels of resistance and that none might become infected if challenge doses very much less than 60 ID50 were used. To prepare the standard inoculation dose, we diluted the commercially available virus preparation (Czech CAPM 351 RHDV, batch 1B, from Elizabeth Macarthur Agricultural Institute, Camden, NSW) with 24 equal parts of sterile PBS. The basic undiluted stock solution has a titre of 3000 rabbit infectious doses/ml so the dose used in our trials was equivalent to a nominal intramuscular dose of 60 ID50 when delivered in a volume of 0·5 ml. Rabbits were inoculated orally, using a 1 ml tuberculin syringe (without needle) to introduce the dose at the corner of their mouths, through the diastemma, and onto their tongues.
Experimental monitoring and sample collection
Experimentally inoculated rabbits were checked for signs of illness (lethargy, ataxia, death) every eight hours (i.e. 07:00, 15:00, 23:00 hours) for 6 days then daily until 14 days post-inoculation. This 8-h time interval allowed the best compromise between reducing stress on the animals caused by human presence while still enabling the collection of relatively fresh samples (e.g. blood for virus assay) and for the time to death to be calculated with reasonable accuracy. In the latter case, rectal temperature was taken immediately when a dead rabbit was found and time to death calculated using equation (1) (below). This equation was previously derived by taking hourly rectal temperature recordings from five rabbits euthanized after experiments and held in the same plastic trial boxes and climate-controlled rooms as used for experiments.
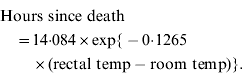
Experimental trials were terminated on day 14 (336 hours) when blood samples were collected from all surviving rabbits. Serum from these blood samples was tested using the array of ELISAs (listed earlier) to determine whether or not the surviving rabbits had seroconverted as a result of exposure to the virus.
Investigating resistance
Each rabbit that survived the initial oral challenge without seroconverting was re-challenged with a 60 ID50 RHDV dose given intramuscularly in the hind leg. Subsequent monitoring and sampling were as for oral challenge. The survival times of rabbits that died were recorded and rabbits surviving to 14 days post-challenge were euthanized and further serum samples obtained for ELISA testing as detailed above.
Analysis of experimental data
Infection rates and survival times of rabbits between groups from each locality were analysed using analysis of variance (ANOVA). However, there were limitations to this approach because survival time and the proportion of rabbits surviving challenge are not really independent variables. For example, where all rabbits survived challenge, no survival time measurements were available and as a consequence the data available for analysis were limited. To make the most of available data, we used non-parametric Kaplan–Meier tests [Reference Kaplan and Meier23] which made pairwise comparisons of the separation of survival curves obtained from each group of rabbits, the shape of each curve being influenced both by the numbers of rabbits that survived the duration of the experiment and the time taken for rabbits that died to succumb to the disease. The XL-STAT-Life package (Addinsoft SARL, Germany) was used for this purpose and the Tarone–Ware test of equality of the survival functions was used for these tests as it was considered to give more conservative probabilities of differences between curves than the related Log-rank and Wilcoxon tests for equality [Reference Tarone and Ware24].
RESULTS
Infection and case mortality rate
Infection rates of rabbits from each site were calculated from the number of rabbits confirmed to have died from RHD plus those few rabbits which survived RHD and seroconverted (determined from blood sample ELISA analysis). Infection rates differed (0·00–0·73) between the rabbit populations tested (Table 1). The null hypothesis that there is no difference between the populations tested can be rejected (ANOVA, G crit=21·104, d.f.=11, P=0·03). Post-hoc analysis using adjusted residuals showed that Bulloo Downs and Yambuk rabbits had significantly higher than expected infection rates, similar to rates observed in unselected domestic rabbits, while Ingliston rabbits had a significantly lower than expected infection rate.
Many (80/86) of the wild rabbits that survived to the end of the 14-day experimental period showed no evidence of infection while only a few (6/86) showed RHDV-specific IgM, IgA and IgG antibodies indicating recovery from infection. Of those rabbits that remained seronegative, many (43%) were subsequently shown to be susceptible to infection (see Intra-muscular re-challenge section below), indicating that they had not become infected at the low dose of virus given. Eighty-seven per cent of those rabbits that became infected with RHDV on initial oral challenge succumbed to acute disease.
Survival times
Survival times of rabbits that died following challenge generally tended to increase in the proportion of rabbits in each group that survived challenge (Table 1). However, differences between populations were not significant (ANOVA: F=1·0606, d.f.=10, P=0·4209). This partly results from the interaction of mortality and survival time because in instances where no rabbits died, or only one or two died, survival time data were unavailable or very limited. Nonetheless, unselected domestic rabbits died on average 59·7 h after oral dosing (95% confidence interval 52·1–81·9 h), whereas the survival times of wild rabbits that died were normally considerably longer, occasionally up to 135 h.
The sex of rabbits did not influence infection rates (ANOVA: F=1·9519, d.f.=1, P=0·1647) or survival times (ANOVA: F=0·5522, d.f.=1, P=0·4620), nor did rabbit body weight influence infection rate (ANOVA: F=0·0018, d.f.=1, P=0·9665) or survival time (ANOVA: F=0·2992, d.f.=1, P=0·5876) (data not shown).
Survival curve analyses
Data from 12 unselected domestic rabbits (three groups of n=4) used as controls during challenge trials on wild rabbits provided a basic survival curve suitable for making comparisons and detecting evidence of resistance in wild rabbit populations. As anticipated from the initial work to establish a suitable experimental challenge dose, eight of the domestic rabbits died from acute RHDV.
Kaplan–Meier analyses showed that survival curves for Bulloo Downs and Yambuk rabbits did not differ significantly from the curve for unselected domestic rabbits, while the survival curves differed significantly for rabbits from the Spring Hill, Michelago, Whetstone, Alice Springs, Yanyanna, Hattah and Ingliston sites (Table 2).
Table 2. The results of Tarone–Ware test of equality of the survival distribution functions (d.f.=1) for each combination of sites. Survival distribution functions from all sites but Yambuk and Bulloo Downs differed from that of domestic rabbits

Bold values denote P<0·05.
Intramuscular re-challenge
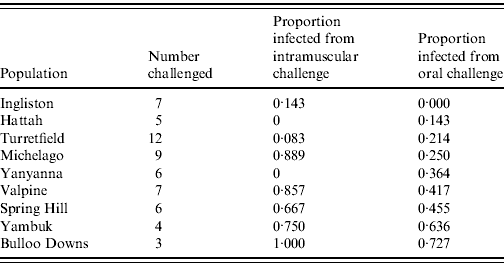
Sixty rabbits that survived the initial oral RHDV challenge without seroconverting were subsequently re-challenged with an equivalent intramuscular dose of 60 ID50 RHDV. Twenty-six (43%) became infected and only one (from Ingliston) survived (Table 3). The remaining 57% of rabbits again showed no evidence of seroconversion, suggesting that from an immunological perspective they had not encountered the virus. The proportion of rabbits in each group that became infected following intramuscular re-challenge was significantly correlated with the proportion that became infected following oral challenge (F=11·84, d.f.=8, P=0·01). In other words, rabbits from populations that showed resistance to oral challenge also showed resistance to intramuscular challenge.
Table 3. Infection rate of rabbits challenged with a 60 ID50 intramuscular challenge, having failed to be infected with an oral dose
Disease resistance and climatic and geographical factors
The expectation that the presence of non-pathogenic RCV-A1 in high rainfall areas of southeastern Australia should slow the development of resistance was investigated using a statistical regression of the proportion of rabbits from each site that resisted infection against the annual average rainfall at that site. Because there were only a very limited number of sites from which we had RHDV challenge data, we could not consider a range of variables in a wider analysis of climatic explanatory variables. We chose rainfall as a surrogate for the hot, dry-cool, wet trend associated with RHDV effectiveness and the presumed presence of RCV-A1. This is acceptable for sites in inland and southeastern Australia where rains are fairly evenly spread throughout the year and plant growth most commonly occurs in winter but the Townsville population was not included as it is in a tropical, summer rainfall area and its annual average rainfall (1350 mm) was nearly double that of any of the other sites.
Although not negating the idea that RCV-A1 may be an important factor in retarding the development of resistance, we did not find the simple correlation between rabbit resistance and rainfall that we anticipated. We certainly found that resistance to infection was low at high rainfall sites (e.g. Yambuk) but we also found that at some very arid sites (e.g. Bulloo Downs) resistance to infection was equally low. Inspection of the data further suggested that a curvilinear relationship between resistance to infection and annual average rainfall was more likely (Fig. 1); a second-order polynomial [equation (2)] potentially explains more of the variance than a simple linear regression (R 2=0·6415).
where r a is the average annual rainfall (mm). The pattern suggests that resistance is low in wet areas, relatively high in intermediate rainfall areas and declines again in very arid areas.

Fig. 1. The proportion of rabbits that survived oral challenge with rabbit haemorrhagic disease virus without developing antibodies as a function of annual rainfall (R 2=0·6415).
DISCUSSION
On the basis of the experimental results we reject the two hypotheses that there is (a) no difference between infection rates caused by RHDV in wild rabbits and unselected domestic rabbits, and (b) no difference in apparent resistance between rabbits collected from different sites in Australia. In the absence of pre-existing data on RHDV infection rates in different wild rabbit populations in Australia we cannot rule out the possibility that other changes (e.g. genetic drift) might explain the geographical variation in apparent resistance to challenge. This, however, seems highly unlikely given that at sites near Yanyanna in South Australia mortality was extremely high (about 98%) as RHDV first spread [Reference Mutze, Cooke and Alexander3], and wild rabbits from the vicinity of Adelaide (near Turretfield in South Australia) could be experimentally affected with extremely low doses of orally administered RHDV (2–3 LD50 units) [Reference Asgari25].
These data suggest that some rabbit populations now show resistance to infection with low doses of the Czech 351 RHDV initially released in Australia. Rabbits from the Yambuk and Bulloo Downs populations (the wettest and driest sites, respectively) appear to be no more resistant to Czech 351 RHDV than unselected domestic rabbits whereas rabbits from other sites appear to be significantly more resistant to infection (Table 2). The rabbits that became infected in our experimental trials mostly died from RHD, the case mortality rate of 87% being not much below that previously recorded for susceptible rabbits [Reference Lenghaus, Munro and Williams26, Reference Pages-Mante27]. This implied that although rabbit resistance to infection may have increased, those that became infected showed at best only a marginally increased capacity to avoid acute disease. Furthermore, it must be assumed that those rabbits which remained uninfected after challenge had not detected the virus in an immunological sense for they formed no antibodies and many could be subsequently infected with a second virus challenge (as discussed later). Increases in the proportion of rabbits that survived were associated with longer survival times in rabbits that died, although only a few rabbits died more than 5 days after inoculation. Survival times have been shown to be longer for oral infection compared to intramuscular or intra-dermal infection [Reference Cooke and Berman28]. This suggests that uptake across the mucosal barrier delays the onset of illness. If resistance interferes in the infection process across the mucosal barrier, it may lead to an increased time between virus exposure and uptake allowing time for immunological responses to counter infection and facilitate survival.
Importantly, rabbits from populations that showed resistance to oral infection also showed a degree of resistance to equivalent doses of virus when the mucosal barrier was by-passed by giving a second, intramuscular dose of virus (60 ID50). Many again failed to become infected. This implies that the mechanism of resistance is not simply confined to the mucosal barrier but must act across a broader spectrum of tissues or even a whole complex of inter-related defences. The association between reduced infection rates and prolonged survival times also implies a common mechanism such as selection of rabbits with genotypes that not only withstand infection but also show a reduction in the rate at which generalized disease develops.
These observations are particularly significant from a theoretical point of view because it is hard to explain why resistance to infection alone would have a selective advantage in wild rabbits. In field populations, most rabbits aged >1 year have antibodies, indicating that they have recovered from RHDV. This means that resistance must only delay infection and that sooner or later rabbits must either encounter a massive dose of virus (e.g. contact with a cadaver) or encounter a field strain of the virus that has greater capacity to infect them than the Czech strain RHDV used in our trials. Delayed infection would mean that rabbits would be older when they became infected and therefore more likely to succumb to RHD than if they had been infected when very young [Reference Robinson29]. By contrast, if resistance to infection was simply one manifestation of a broader process which slowed the development of generalized disease and gave more time for other defensive antiviral mechanisms to function, the selective advantage would be more evident.
Our evidence of development of genetic resistance to RHDV infection in wild rabbits adds to a growing body of research exploring possible genetic mechanisms to explain infection processes and disease behaviour. It has been shown that histo-blood group antigens (HBGAs) on mucosal cells bind both RHDV [Reference Ruvoën-Clouet30] and human norovirus [Reference Lindesmith31] and, in the latter case, resistance to infection has developed through non-functional mutations that inhibit the expression of HBGAs on mucosal cell surfaces [Reference Le Pendu32, Reference Thorven33]. However, this does not appear to be the case for RHDV and it has recently been shown that alleles of the fucosyltransferase (Fut-2) and secretor (Sec-1) genes that help to determine HBGA expression are functional [Reference Guillon34]. It has further been shown that although HBGAs play an undefined role in the infection process (e.g. they are not present on hepatocytes), the phenotypic expression of HBGAs still influences infection outcomes [Reference Nyström35] because RHDV from any given strain binds better to the cells of some rabbits than others. In a specific investigation of Australian wild rabbits it was shown that Czech 351 virus particles are able to bind to B as well as H type-2 surface antigens but have no ability to bind to A surface antigens. This means that the current field strains of RHDV in Australia, all derived from Czech 351 virus, should positively select A+B– phenotypes as appears to be the case in the most heavily selected populations. At Hattah, where rabbits are at best only weakly protected by the presence of non-pathogenic RCV-A1, A+B– rabbits make up 33% of the population whereas at Spring Hill (near Bendigo) and Ingliston (near Bacchus Marsh) where RCV-A1 is present, A+B– rabbits make up only 9% and 4% of the populations, respectively [Reference Nyström35].
However, our observations with wild rabbits inoculated intramuscularly to by-pass the mucosal barrier suggest that resistance to RHDV infection depends on factors in addition to binding to HBGAs on the mucosal cell surface. This does not rule out the possibility that the HBGAs may regulate virus spread within the rabbit as well as attachment to mucosal surfaces but, nonetheless, suggests that other mechanisms to counter RHDV infection are likely.
We proposed that genetic resistance to infection with Czech 351 might develop slowly in high rainfall areas because of the presence of non-pathogenic RCV-A1 which protects rabbit populations from the full impact of RHD. However, we found this theory could not be verified because we failed to readily establish a significant inverse relationship between rabbit resistance to infection and high rainfall sites where RCV-A1 was most prevalent. Inspection of the data suggest that genetic resistance to infection was low in high rainfall areas but this was further complicated by the observation of very low resistance in rabbits from very arid areas as well. While this information cannot be taken further on the basis of our limited dataset, it would be an idea worth following up because genetic resistance to myxoma virus infection also developed most rapidly in regions of moderate rainfall [Reference Parer11] raising the possibility that the rate of evolution of resistance may be influenced by other factors (e.g. rabbit population productivity) and not just interactions between virus and host alone.
The apparent lack of discernible difference between rabbits from some wild populations and the domestic controls also poses interesting questions. Given that protection afforded by antibodies to RCV-A1 is not complete and that field doses of RHDV appear to kill only about 50% of rabbits protected in this way [Reference Strive20, Reference Mutze36], a complete absence of selection for resistance would seem likely only if RHDV was prevented from spreading in areas where RCV-A1 most commonly occurs. In fact this may well be the case. At Cattai, for example, RHDV failed to establish despite repeated releases [Reference Richardson5] and at Kojaneerup in southwestern Western Australia RHDV initially spread then failed to persist on a wide scale [Reference Bruce, Twigg and Gray37]. Only 1% of rabbits at Kojaneerup showed evidence of RHDV infection in the following years, although most had antibodies indicative of the presence of a non-pathogenic lagovirus [Reference Bruce and Twigg18, Reference Bruce, Twigg and Gray37, Reference Bruce and Twigg38]. Sites where RHDV could be blocked in this way are in coastal regions with relatively high average annual rainfall, e.g. 800 mm for Cattai [Reference Richardson5], and 485–690 mm for southwestern Western Australia [Reference Bruce, Twigg and Gray37]. The Yambuk site sampled in this study fits this pattern too, being situated on the coast with an average annual rainfall of 770 mm. At such sites it seems that RCV-A1 might inhibit RHDV so strongly that there is little evidence of natural selection for resistance. The low resistance shown in rabbits from sites of very low rainfall is more difficult to explain. In his considerations of selection for resistance to myxomatosis, Rendel [Reference Rendel39] suggested that where disease caused very high mortality, so few recovered rabbits were recruited that they contributed little to the genetic make-up of the adult population.
In parallel with our understanding of the co-evolution of myxoma virus virulence and rabbit resistance, where a long-term biological arms race seems to be underway [Reference Fenner and Fantini1], the suggestion that rabbits are developing resistance to RHDV infection is only a first step. Czech 351 RHDV has shown steady nucleotide sequence changes and there are now a number of distinct field variants derived from the virus originally released in Australia (J. Kovaliski, unpublished observations). This makes it important to ask if RHDV is co-evolving to maintain relatively high virulence as rabbit resistance grows. If this proves to be the case, it may yet turn out to be a long-lasting bio-control agent after the style of the myxoma virus. Nonetheless, because rabbits are regaining numbers in some areas, it seems that even if a dynamic equilibrium between rabbit resistance and virus virulence is being forged, it will not hold rabbits at levels low enough to permanently avert ecological and economic damage.
ACKNOWLEDGEMENTS
We gratefully acknowledge Steve McPhee, Greg Mutze, Dr Ron Sinclair, Dr Maija Marsh and Dr Bill Lowe for supplying rabbits. We thank Dr Lorenzo Capucci and the Istituto Zooprofilaticco Sperimentale in Brescia, Italy, for providing ELISA reagents and advice. David Aster, Dallas Powell, Glen Rettke and Brian Koina maintained the rabbits and facilities for experimentation. Drs Stephen Sarre, Brett Lidbury, Dane Panetta and David Berman provided project advice and reviewed the manuscript. Funding was received from: Australian Wool Innovation, Invasive Animals Cooperative Research Centre, Institute for Applied Ecology at the University of Canberra and the Queensland Department of Employment, Economic Development and Innovation. This work was done in part fulfilment of a Ph.D. study through the University of Canberra.
DECLARATION OF INTEREST
None.






