INTRODUCTION
Q fever is a zoonosis caused by Coxiella burnetii, an intracellular Gram-negative bacterium that is prevalent throughout the world [Reference Raoult, Marrie and Mege1]. Domestic ruminants are considered to be the main reservoir for Q fever in humans [Reference Woldehiwet2], although other animal species, including pet animals, birds and reptiles, may also be responsible for human cases. Transmission to humans is mainly accomplished through inhalation of contaminated aerosols. The main clinical symptom of Q fever in goats and sheep is abortion and in cattle reduced fertility. With abortion, 1 000 000 000 C. burnetii/g placenta can be excreted [Reference Arricau Bouvery3]. Duration of shedding of C. burnetii by infected livestock varies depending on the excretion route and species. In milk, C. burnetii can be excreted for 8 days in ewes and up to 13 months in cattle. In faeces, C. burnetii can be excreted up to 8 days after lambing in ewes and up to 20 days in goats [Reference Arricau-Bouvery and Rodolakis4]. Goats may shed C. burnetii in two successive kidding periods [Reference Berri5]. Most animal species carrying C. burnetii show no symptoms at all [Reference Arricau-Bouvery and Rodolakis4]. In humans infection with C. burnetii remains asymptomatic in ~60% of infected persons. In symptomatic patients, acute Q fever usually presents as a flu-like, self-limiting disease, atypical pneumonia or hepatitis. Infection in pregnancy may lead to adverse pregnancy outcomes, such as spontaneous abortion or premature delivery. About 1–5% of all Q fever cases may progress into a chronic infection, often leading to life-threatening endocarditis [Reference Arricau-Bouvery and Rodolakis4, Reference Fenollar, Fournier and Raoult6–Reference Musso and Raoult9].
Since 2007, a Q fever outbreak has been ongoing in The Netherlands and this is referred to as the largest outbreak of Q fever ever reported in the literature [Reference Schimmer10]. However, Q fever is not an entirely new disease in The Netherlands. In this review we give an overview of the history of Q fever in The Netherlands, both in animals and in humans, the emergence of the disease and the control measures that have recently been taken. In conclusion, we put the outbreak in an international perspective.
Q fever becomes endemic, 1956–2005
Situation of Q fever in humans between 1956 and 2005
In the 1950s a comprehensive survey of the global distribution of Q fever was commissioned by the World Health Organization. In The Netherlands, between 1951 and 1954, almost 10 000 human sera were tested for Q fever with a complement-fixation test and all were negative [Reference Kaplan and Bertagna11, Reference Wolff and Kouwenaar12]. In 1956, the first three human cases were reported in The Netherlands [Reference Westra, Lopes Cardozo and Ten Berg13]. Two patients might have been linked to imported cattle or a visit abroad, while in the third patient there was no obvious cause. In 1958 and 1967 two human Q fever cases were described associated with the handling of imported wool [Reference Jordans14, Reference Terwindt and van Holten15].
The notification of infectious diseases started in The Netherlands in 1865. The law changed several times and the list of notifiable diseases became longer with every change. Q fever was made notifiable in 1975, although it was a very rare disease at that time [Reference van Vliet16]. Between 1975 and 2006 the annual number of cases increased from 0 to 32 per year (Fig. 1). Thirty-three of the cases notified between 1979 and 1983 were investigated more thoroughly. Twenty-two (67%) of these patients were probably infected in The Netherlands through contact with animals or animal products [Reference Richardus17]. A survey in 1982/1983 among 432 persons, considered to be at high risk because of close contact with animals and animal products, showed high percentages (58% in taxidermists to 84% in veterinarians) of seropositive responders with mainly IgG antibodies against C. burnetii phase-II antigen, indicating that Q fever had become endemic in The Netherlands [Reference Richardus18].
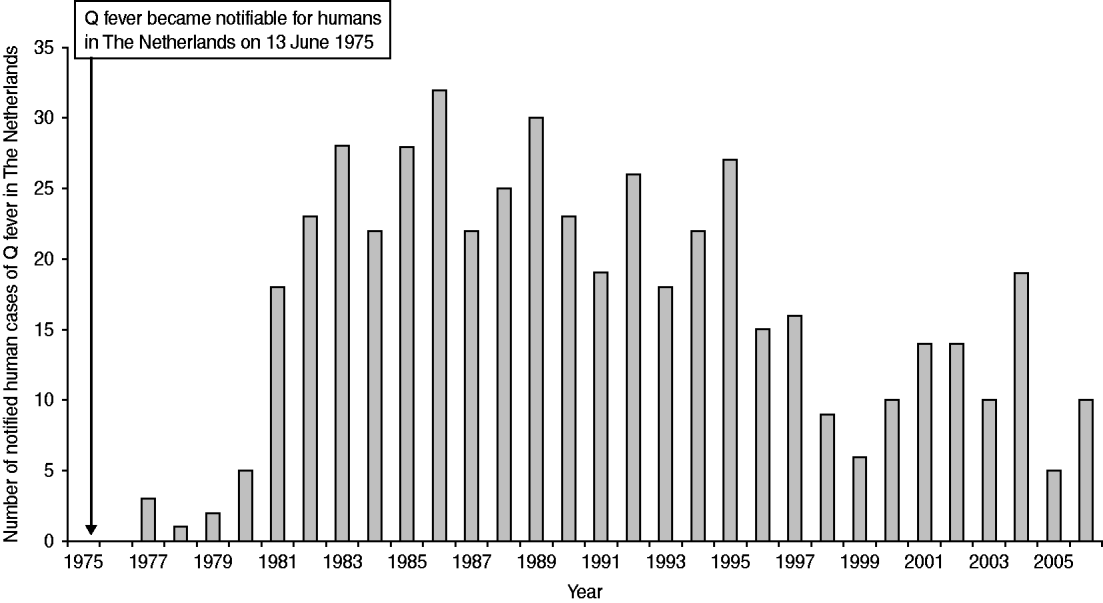
Fig. 1. Number of notified human cases in The Netherlands between 1975 and 2006.
The increase in human cases between 1977 and 1983 (Fig. 1) was assessed by Richardus et al. [Reference Richardus18] by testing serum samples from persons, not considered to be at high risk, taken in 1968, 1975, 1979, and 1983 with an indirect immunofluorescence test (IFT) for specific IgG antibodies against C. burnetii phase-II antigen. In adults an average seroprevalence of 46% in 1968 and of 48% in 1983 was found. In children seroprevalences varied from on average 54% in 1975 to 28% in 1979 and 1983. These results showed no significant increase in the percentage of infected persons over the years 1968–1983. The increased number of notified cases since 1980 was explained by the introduction of a sensitive indirect immunofluorescence test for IgM antibodies against C. burnetii. In the same study [Reference Richardus18] occupational groups with a high risk of infection showed a significantly higher proportion of seropositives compared to the low-risk control group, in 1983 84% of 221 veterinarians and 68% of 94 residents of dairy farms were positive, whereas in the control group on average 29% tested positive for IgG antibodies against phase II of C. burnetii. The age distribution suggested an early onset of infection as antibody percentages over all age groups between 1 and 64 years were comparable [Reference Richardus18]. It remains unclear why seroprevalence in the non-high-risk groups, varying between 30% and 50% in the 1980s, was high while on average 23 clinical Q fever cases were detected. Protective immunity from childhood [Reference Richardus19] and under-diagnosis [Reference Gageldonk-Lafeber20] could have played a role, but the serological test methods that were used in the 1980s were developed in-house and are no longer available, so the sensitivity and specificity of these tests can no longer be verified.
Situation of Q fever in animals between 1956 and 2005
Surveys in the early 1950s indicated that the bovine population was free of Q fever at that time [Reference Kaplan and Bertagna11, Reference Wolff and Kouwenaar12]. Between 1981 and 1987 the endemic state of Q fever in farm animals was confirmed by seroprevalence studies in cattle, sheep and goats [Reference Houwers and Richardus21, 22]. In 1981, 55% of 20 sampled cattle on one farm were tested positive. In 1987, 10% of 1320 non-dairy cattle, 21% of 1160 dairy cattle and 3% of a total of 494 dairy heifers were seropositive. At the herd level, about 36% were found positive with an average of 35% seropositive animals per herd. The occurrence of C. burnetii in cattle herds seemed to be associated with abortions, as 77% of the herds with abortions had a mean seroprevalence for Q fever of 39%. For the 31% of the herds without abortions, the mean seroprevalence was only 17%. In sheep 3·5% (127/3603 sheep sera from 191 flocks) of the animals were found positive with a herd prevalence of 27%. In goats 2/594 sera from individual goats from 54 flocks were positive. In 1992 prevalence in 220 representative cat sera was 10% and in 400 representative dog sera 13% (D. J. Houwers, et al. unpublished data).
Transition period, 2005–2007
Changing situation of Q fever in animals since 2005
Clinical Q fever in animals was diagnosed in The Netherlands for the first time in 2005 [Reference Vellema23]. In two dairy goat herds with abortion problems C. burnetii was detected by immunohistochemistry (IHC) on placentas [Reference Wouda and Dercksen24]. From 2005 to 2007 Q fever abortions were diagnosed on 15 dairy goat farms and one dairy sheep farm (Table 1). Abortions, with herd rates up to 60%, were seen mainly in the final month of pregnancy without signs of general illness, although some goats were temporarily a little sluggish with reduced appetite. After abortion some goats showed symptoms of endometritis. Full-term kids were weak, with low body weight and high mortality. In several apparently healthy kids the rearing period was complicated by respiratory and digestive tract disorders. Treatment of pregnant goats with oxytetracyclines did not reduce the abortion rate [Reference Wouda and Dercksen24]. Retrospectively, C. burnetii could be detected by IHC in one preserved dairy goat's placenta from a farm with abortion problems in 2001. In dairy cattle herds C. burnetii antibodies were detected on 57% of 344 farms using ELISA on bulk tank milk (BTM) samples during 2005–2006. It was calculated that on 35% of the farms at least 30% of the cattle could be positive [Reference Muskens, Mars and Franken25].
Table 1. Number of dairy goat and dairy sheep farms with confirmed Q fever abortions

* Including one farm with animals at two locations.
Changing situation in humans since 2007
In 2007, individual human Q fever cases were reported, occurring after visits to dairy goat farms with abortion problems [Reference Wouda and Dercksen24, Reference Weevers26]. The first documented outbreak of Q fever in The Netherlands was described by Karagiannis et al. [Reference Karagiannis27] and Van Steenbergen et al. [Reference Van Steenbergen28]. A total of 168 human cases were notified in 2007 (Fig. 2). The epidemic became apparent by a number of indicators: first, a medical microbiologist reported two patients admitted to hospital with severe pneumonia, who did not respond to standard antibiotic therapy. Four days later, a general practitioner informed the Municipal Health Service (MHS) of an unusual number of ten patients with atypical pneumonia in his practice. A similar report by another general practitioner from the same area was received 2 weeks later. Finally, an increase in the number of notifications for Q fever was noticed in the national registration system of the National Institute for Public Health and the Environment (RIVM). No specific source could be identified, but it was postulated that the high number of abortions on the surrounding dairy goat farms might be the source of the human Q fever cases in the province of Noord-Brabant. Airborne transmission of contaminated dust particles could have been facilitated by the unusually hot and dry weather in the spring of 2007.
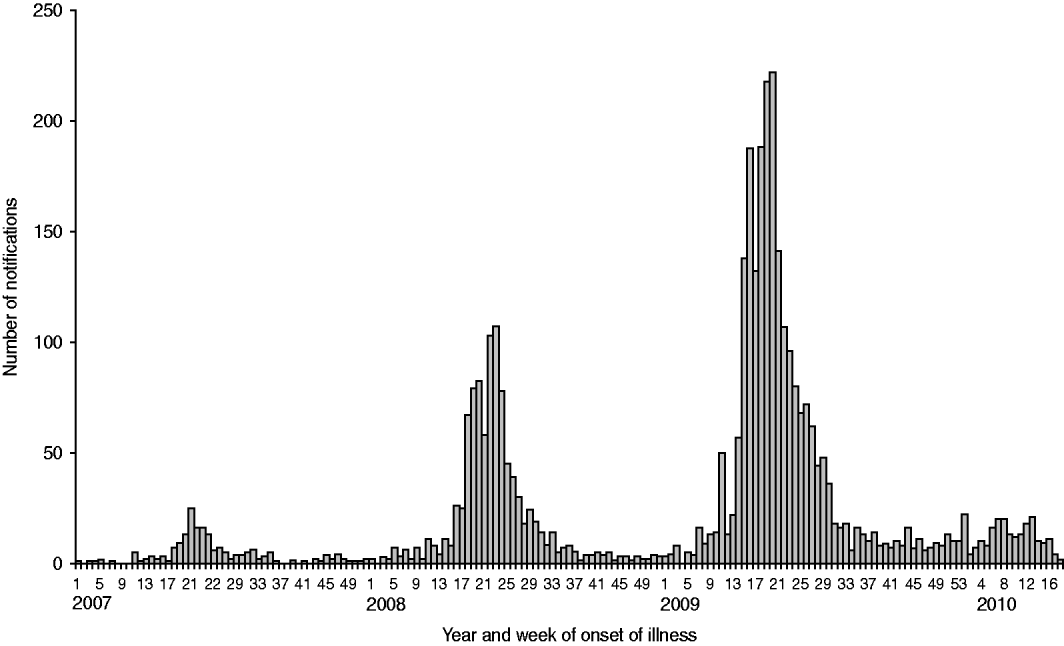
Fig. 2. Number of notified human Q fever cases with a known first day of illness according to the week of onset of symptoms, from 1 January 2007 to 11 May 2010. 2007 (n=168), 2008 (n=1000), 2009 (n=2355), 2010 (n=208). Total number of human cases in 2007–2009 (n=3523) (compiled by F. Dijkstra).
In 2007, the outbreak was concentrated around a single village and in this village a case-control study was performed [Reference Karagiannis29]. Contact with manure, hay, and straw proved to be a risk factor. Moreover, people living in the eastern part of the village close to ruminant farms, of which one dairy goat farm had a recent history of abortion problems, were at higher risk than people living in other parts of the village. Contact with animals and consumption of raw milk products were not significant risk factors in the multivariable analysis.
Notification criteria for a confirmed human Q fever case were a clinical presentation with fever or pneumonia or hepatitis and confirmation of the diagnosis in the laboratory by at least a fourfold rise in IgG antibody titre against C. burnetii in paired sera or the presence of IgM antibodies against phase II or antibodies against C. burnetii phase I [30]. A probable case was defined as clinical signs with a single high antibody titre [Reference Schimmer31].
Response phase, 2008–2010
Continuation of the human outbreak
In 2008, it soon became clear that the 2007 outbreak was not an isolated incident. In May 2008 an outbreak of Q fever occurred in a psychiatric care institution in Nijmegen, province of Gelderland, ~15 km from the 2007 outbreak area [Reference Koene32]. At least 28 in-patients, employees, and visitors had laboratory-confirmed Q fever illness and several patients of the institution developed atypical pneumonia. A small flock of sheep without clinical symptoms of Q fever was present at the location. Patients had close contact with lambs, including cuddling as part of the patients' therapy sessions. Furthermore, on a dairy goat farm close to the city of Nijmegen, a large number of goats unexpectedly aborted their offspring. Q fever was confirmed by PCR on vaginal swabs. The farmer showed no clinical symptoms indicative for Q fever. The farmer's wife suffered from only moderate flu-like symptoms, including fever and coughing, and for both persons Q fever was confirmed by PCR on serum, throat swabs, urine and faecal samples. To determine the genetic relatedness between the human and animal clinical samples from both locations multiple-locus variable number tandem repeat analysis (MLVA) was carried out. Clinical samples from other patients from different locations in the same high-risk area were included in the study. All genotypes showed a high degree of similarity with most genotypes differing from each other by only a single marker, suggesting a clonal origin (J. J. H. C. Tilburg & C. H. W. Klaassen, unpublished observations) [Reference Klaassen33, Reference Tilburg34].
Eventually, 1000 human Q fever cases were notified in 2008 (Fig. 2). The centre of the outbreak and the area with the most human cases were the same as in 2007, but there was a clear geographical spread to adjacent areas (Fig. 3). The age distribution (range 7–87 years, average 51 years) was similar to 2007, but the hospitalization rate decreased from 50% in 2007 to 21% in 2008 [Reference Schimmer10, Reference van der Hoek35]. The high hospitalization rate of 50% in 2007 might be biased by active case-finding in a retrospective survey among hospitalized cases [Reference van der Hoek35]. The overall gender breakdown was the same in 2008 as in 2007: the female to male ratio was 1:1·7 [Reference Schimmer10].

Fig. 3. Map of The Netherlands. Left: Number of human cases in 2007 and 2008. The red line shows the dairy goat and dairy sheep voluntary vaccination area in 2008. Right: Dairy goat farms and dairy sheep farms with Q fever abortion history between 2005 and 2008. [Compiled by National Institute for Public Health and the Environment (RIVM) and Animal Health Service (GD).]
In April 2009 a sharp increase in human cases was observed again resulting in a total number of 2355 cases (Fig. 2). Again most of the human cases were from the same area as in 2007 and 2008, with yet again a wider geographical spread (Fig. 4). Pneumonia is the predominant presentation of Q fever in The Netherlands. For patients notified in 2008 for whom clinical details were available, 545 were diagnosed with pneumonia, 33 with hepatitis and 115 with other febrile illness [Reference Schimmer31]. The age median of 50 years in 2009 did not differ from that in 2008, neither did the hospitalization rate of 20% nor the gender break down of 1:1·7 female to male ratio [Reference van der Hoek35].
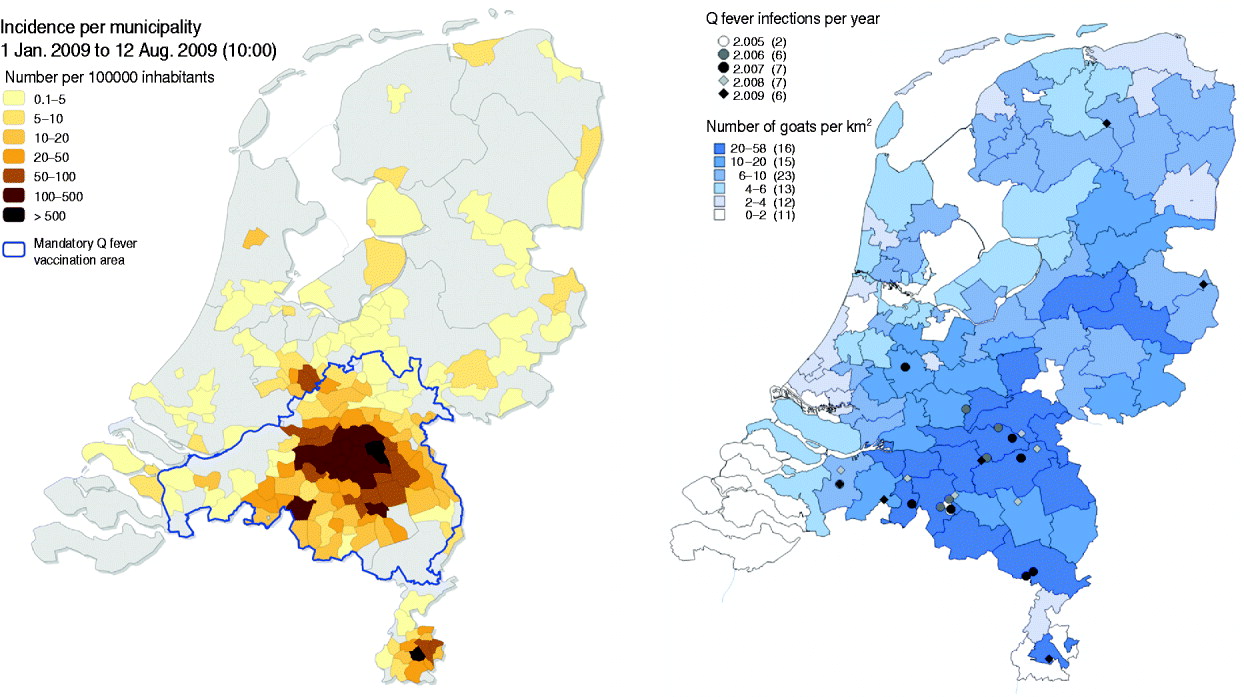
Fig. 4. Map of The Netherlands. Left: Human Q fever incidence/100 000 inhabitants per municipality in 2009. The blue line shows the dairy goat and dairy sheep mandatory vaccination area in 2009. Right: Dairy goat farms with Q fever abortion history between 2005 and 2009. Darker blue indicates more goats/km2. [Compiled by National Institute for Public Health and the Environment (RIVM) and Animal Health Service (GD).]
The overall outbreak in humans consisted of at least 10 separate clusters with multiple sources of exposure. One cluster became apparent in 2008 with a strong connection to one goat farm with abortion problems. Patients were living downwind of the goat farm. Living within 2 km of the goat farm was associated with a higher risk of Q fever infection compared to living >5 km from the farm [Reference Schimmer36]. In 2009, no abortions were notified on this farm, nor in this area, and veterinary measures such as the handling of manure, hygiene measures and a visitors ban were implemented (measures are clarified in the section ‘Response in the veterinary field’), but the number of human cases still increased. To date, the source of this increase in 2009 remains unclear [Reference Schimmer31]. In general, 59% of the notified human cases in 2009 lived within a 5-km zone around a notified dairy goat or dairy sheep farm, while 12% of the Dutch population live within such zones [Reference van der Hoek35].
In 2010 criteria for detection of C. burnetii in blood, serum or a sample from the respiratory tract were added to the notification criteria for human Q fever cases. In addition, only acute cases with the day of first illness within 90 days of notification are recorded in the national infectious disease notification databases [37].
During the period January to May 2010, 208 human cases were registered which indicates a decrease in the number of notified human cases compared to 2009 (Fig. 2). Although weather conditions may have been unfavourable for transmission, it is hoped that the decrease in the number of human Q fever cases was caused by reduced exposure due to veterinary interventions.
Dairy goat industry and prevalence of Q fever in small ruminants
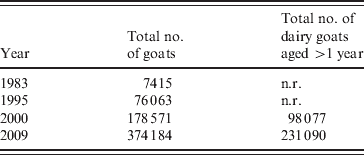
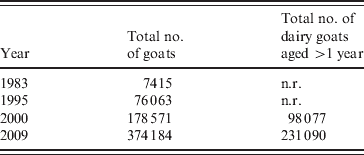
The dairy goat industry in The Netherlands is concentrated in the province of Noord-Brabant, with farm sizes ranging from 300 to 7000 goats with an average of at least 600 animals in 2007. Goat density was 38·1 goats/km2 [Reference Roest38]. These farms often bordered close to villages and cities. The total number of registered small ruminant farms in The Netherlands in 2008 was 52 000. The number of professional dairy goat farms with more than 200 adult goats was 350 and the professional dairy sheep industry consisted of 40 farms [Reference Van den Brom and Vellema39]. Dairy goat farming in The Netherlands started after the introduction of the European milk quotation system for dairy cattle in 1984 and increased after the outbreaks of classical swine fever in 1997 and foot-and-mouth disease in 2001. The total number of goats increased from 7415 in 1983 to 178 571 in 2000 and to 374 184 in 2009. The total number of dairy goats aged >1 year increased from 98 077 in 2000 to 231 090 in 2009 (Table 2) [40, 41].
In 2008, nationwide Q fever seroprevalence in all small ruminants was low; only 7·8% of goats and 17·8% of goat farms were positive, and 2·4% of sheep and 14·5% of sheep farms were positive. In 26% of the BTM samples from 306 dairy goat and dairy sheep farms C. burnetii DNA could be detected. Analysis of the first 13 goat farms with abortions showed an average number of goats per farm of 900 of which 20% aborted. The average number of sheep on the two affected dairy sheep farms was 400 with an abortion rate of 5% [Reference Van den Brom and Vellema39].
Response in the veterinary field
In June 2008, Q fever became notifiable for small ruminants kept for milk production following the advice of experts (Table 3). Additional measures were taken to reduce the assumed risk associated with the spread of manure and to restrict the number of visitors to infected farms. In October 2008, voluntary vaccination of goats was made possible by the Ministry of Agriculture in the high-risk Q fever area in Noord-Brabant with the so far unregistered phase-I Q fever vaccine for ruminants [Coxevac®, Ceva Santé Animale, France; Fig. 3 (red line), Table 3] [42]. A total of 36 000 goats were vaccinated in an area within a radius of 45 km around the village of Uden. This was the first time a Q fever vaccine had been used with the ultimate goal of reducing the number of human Q fever cases. In order to reduce the number of human cases the exposure of humans to C. burnetii should be reduced. To achieve this, excretion of C. burnetii from the animal host should be minimized, particularly by the prevention of abortion due to Q fever. Phase-I Q fever vaccines, contrary to phase-II vaccines, strongly reduce the number of abortions and excretion of C. burnetii in challenged pregnant goats that were initially Q fever-negative [Reference Arricau-Bouvery43]. In clinically Q fever-infected goat herds vaccination with a phase-I vaccine should reduce the excretion of C. burnetii, especially in young animals vaccinated before the breeding season [Reference Rousset44]. In general, vaccination with a phase-I Q fever vaccine is expected to be effective in non-infected goats. The effect of vaccination in Q fever-infected goats is not clear and may imply a continuation of the risk of shedding C. burnetii and exposure to humans. According to the summary of product characteristics (SPC), the phase-I Q fever vaccine is not indicated for use in pregnant sheep. Assessment of the efficacy of the phase-I vaccine in pregnant cattle showed a similar probability of the animals becoming shedders when vaccinated while pregnant compared to non-vaccinated animals [Reference Guatteo45].
Table 3. Overview of legislation concerning Q fever in small ruminants in The Netherlands [42]
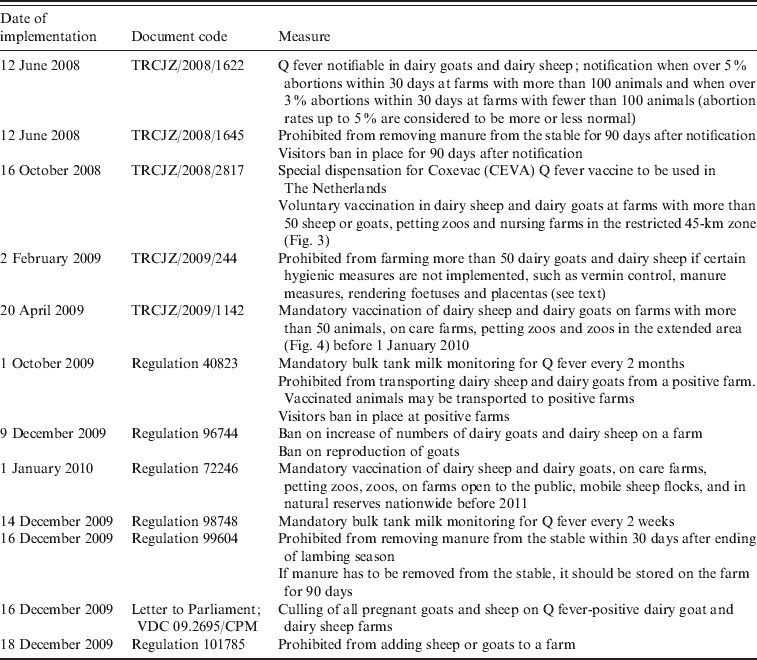
In February 2009, measures taken by the government were tightened with a stringent hygiene protocol made mandatory for all professional dairy goat and dairy sheep farms in The Netherlands, independent of their Q fever status. The hygiene protocol included vermin control, measures for handling manure (farmers were not allowed to remove manure from their deep litter stables for at least 1 month after the kidding season, were obligated to cover manure during storage and transport, and had to underplough manure immediately when spreading on farming land or had to store it for at least 3 months), compulsory rendering of aborted foetuses and placentas and improved general farm hygiene (such as the prevention of dust and aerosol formation, protective industrial clothing, clean delivery equipment and the use of sufficient high-quality bedding material). In addition, farmers were advised to submit aborted foetuses for pathological examination [Reference Van den Brom and Vellema39, 46]. Vaccination became mandatory for all dairy goats and dairy sheep, and the vaccination area was extended from the 45-km zone around Uden in 2008 (red line in Fig. 3) to the whole province of Noord-Brabant and small neighbouring areas in 2009 (indicated by the blue line in Fig. 4).
In response to the increasing number of human cases in 2009, additional measures were implemented in October 2009: PCR positivity of BTM on dairy goat and dairy sheep farms became a notification criterion of Q fever in small ruminants in addition to unexpectedly high abortion rates (>5%); a transport ban of animals from Q fever-positive farms and a visitors ban at Q fever-positive farms (Table 3). Improved monitoring of farm infection status with BTM monitoring was advised by experts. The background of this advice was that the abortion rate is difficult to measure in a flock with over 600 animals, and on infected farms C. burnetii can also be excreted in large quantities during normal birth. In large infected herds with many animals having normal deliveries, the total amount of excreted bacteria can also be very high with a subsequent risk for public health. BTM monitoring could give insight to the number of farms where C. burnetii is present. Initially, BTM monitoring was take place every 2 months, but with the increased awareness of the risk of Q fever and the possible risk that Q fever-positive farms might pose during kidding season, the frequency of monitoring was increased to every 2 weeks. Due to BTM monitoring the number of Q fever-positive dairy goat and dairy sheep farms had increased to 88 on 17 April 2010 (Fig. 5).

Fig. 5. Map of The Netherlands with all 88 bulk tank milk-positive dairy goat and dairy sheep farms known on 17 April 2010. The red zone is the 5-km zone around a positive farm. [Compiled by Dutch Ministry of Agriculture, Nature and Food Quality (LNV).]
With changing public and political awareness of Q fever, the Outbreak Management Team or expert panels advised the Ministries of Health and Agriculture about additional risk reduction measures. At a meeting in December 2009, experts stated that they expected vaccination would not be sufficiently effective in reducing the excretion of C. burnetii in the 2010 kidding season, due to the fact that not all dairy goats and dairy sheep had been vaccinated before the 2009 breeding season. A more considerable reduction in the possible excretion of C. burnetii and thus, environmental contamination, thereby attempting to reduce human exposure and potential risk to public health in 2010, might be achieved by preventing pregnant Q fever-positive goats on Q fever-positive farms from kidding. These animals were identified as high-risk animals. Veterinary experts from the national veterinary reference institute (the Central Veterinary Institute of Wageningen UR), taking consideration of the findings of Rousset et al. [Reference Rousset47], believed it impossible to distinguish infected pregnant animals from non-infected pregnant animals by laboratory testing prior to the start of the kidding season. This was the basis of the decision to cull all pregnant animals on Q fever-positive farms. In addition, breeding dairy goats and dairy sheep was prohibited until at least June 2010. The culling started in late December 2009 and was completed in June 2010, when a number of temporary animal measures imposed by the government ended and now require revision.
The measures in the veterinary field were taken to identify risk farms and to reduce the excretion of C. burnetii from these farms. Although the effectiveness of certain measures is still unclear and the lag time of the measures and the contribution of environmental contamination with C. burnetii to the exposure of humans is unknown, the decreasing numbers of human cases in 2010 thus far might indicate that the measures taken since 2008 have been effective.
The Dutch Q fever epidemic in perspective
Q fever was present in The Netherlands long before it became a problem in public health and animal husbandry. In this period, Q fever appeared to be neither a major health problem for humans, or for domestic animals. This situation, with high seroprevalence in animal populations but few human cases, exists in most European countries [48]. In the years 2005 and 2006, Q fever became a problem in the dairy goat and dairy sheep industry. Abortion storms in small ruminants were not confirmed prior to 2005, although abortions with unknown aetiology are part of small ruminant husbandry with abortion rates up to 5%. It is not clear why Q fever became a major problem in The Netherlands but not elsewhere. Several factors could have facilitated a change in epidemiology in goats: first, an increase in goat density in specific areas of The Netherlands and second, extension of the farms over the years. These two factors could have affected in-herd and between-herd dynamics of Q fever, resulting in outbreaks. Third, there could be pathogen-related factors with circulation of a highly virulent C. burnetii strain. In the years 2007 and 2008, it became clear that Q fever also posed a problem to public health. The connection between Q fever problems in the dairy goat and dairy sheep industry and in the human population was made by several authors based on epidemiological and C. burnetii typing-based findings [Reference Van Steenbergen28, Reference Klaassen33, Reference van der Hoek35]. The increase in goat density took place in the highly populated province of Noord-Brabant (average population density in Noord-Brabant is 497 inhabitants/km2, compared to an average of 398 inhabitants/km2 for The Netherlands). This proximity to a source excreting high numbers of C. burnetii during abortion, with transmission facilitated by dry weather and high numbers of susceptible humans is probably the main cause of the human Q fever outbreak in The Netherlands. The relationship between the change in epidemiology of Q fever in humans and changes in animal husbandry has been shown earlier. After the collapse of the large state farms in Bulgaria in the 1990s individual farmers started to raise goats, which resulted in a more than doubling of the number of goats to 1 million in 7 years [Reference Serbezov49]. This increase, together with a change to a more extensive husbandry system, was believed responsible for the increase of human cases. The increase in human Q fever in Germany was also probably related to socio-geographical factors associated with urbanization of rural areas [Reference Hellenbrand50]. Despite these examples no general conclusions can be drawn, as C. burnetii is endemic in domestic animals throughout Europe and infection can be maintained in a wide range of husbandry systems. Although risk factors, such as an association between human infection and small ruminants, the proximity of animals (especially during parturition) and human populations, and specific weather conditions are clear, there is still an incomplete understanding of transmission pathways with regard to the maintenance of Q fever within the animal reservoir and its transmission to humans [48]. In addition, to date there are no indications of Dutch Q fever spreading to adjacent areas in Belgium and Germany [Reference van der Hoek35].
The question can be raised if the Dutch outbreak is indeed the largest of its kind. A posteriori, it would be difficult to establish if the current epidemic in The Netherlands, with 3523 human cases within three consecutive years, represents a unique phenomenon. In Paragyurische in Bulgaria, more than 2000 cases that were probably due to Q fever were diagnosed in a 6-month episode in 1993. Although confirmation of Q fever was hampered, these numbers of patients were comparable to the 2355 in 2009 in The Netherlands. The Q fever outbreak of the Balkans, named ‘Balkangrippe’, during the Second World War was at least comparable in size. In 1941 over 1000 cases were reported among German troops and during the years 1942–1945 comparable outbreaks were reported in general terms [Reference Spicer51]. However, in these epidemics, no systematic investigations were performed. The importance of this in combination with the availability of diagnostic tests was also shown in the epidemic in a Swiss Alpine valley in 1983 [Reference Dupuis52]. The hospitalization of seven patients with atypical pneumonia was able to be diagnosed as due to Q fever as a result of newly available diagnostic tests. This detection was followed by a very large retrospective study in which infection was identified in nearly 15% of the inhabitants of the valley, probably correlated with migration of sheep. This showed that the proportion of patients presenting sufficiently severe symptoms to be hospitalized accounted for only 2%, and that 56% were completely asymptomatic. This means that for each patient being diagnosed, an additional 50 patients probably remain undiagnosed. This is solely based on systematic testing of the hospitalized patients with fever. Under these conditions, it is very difficult to evaluate the true incidence of Q fever, and it is highly likely that some epidemics have gone completely unnoticed. Thus, the reported incidence of Q fever depends on three distinct elements. First, it depends on the true incidence of the disease including existence of epidemics. Second, the reported incidence depends on sensitivity and specificity of the diagnostic tools used. In the Dutch epidemic the use of serological tests, which are now commercially available and the use of real-time PCR on various clinical specimens allowed a better assessment of the incidence. Finally, the interest of the clinicians and the awareness of the general public incontestably reinforces the quality of detection and the percentage of detected patients. With monitoring systems more cases might be detected, but few data exist on large-scale monitoring of Q fever in the human population. In France, preliminary data showed a regular increase in the number of diagnosed cases of acute Q fever which probably testifies to the growing interest and the diagnostic capacities for this disease. Retrospective research in The Netherlands also shows that real-time syndrome surveillance might have detected clusters of human Q fever cases up to 2 years earlier than 2007 [Reference van den Wijngaard53].
CONCLUSION
The Q fever epidemic in humans in The Netherlands arose from an endemic state and was preceded by severe Q fever abortion problems in dairy goats and dairy sheep. Dairy goats and dairy sheep are considered to be the main source of human outbreaks and control measures were focused on these animal species. Although it is too early to evaluate the magnitude of the human Q fever epidemic in 2010, the decrease in the number of human cases compared to the previous year is promising. The proximity to small ruminants excreting high numbers of C. burnetii during abortion, with transmission facilitated by dry weather and high numbers of susceptible humans is probably the main cause of the human Q fever outbreak in The Netherlands. Q fever outbreaks can easily be missed in the human field as well in the veterinary field. In general good monitoring and surveillance systems are necessary to assess the real magnitude of Q fever.
ACKNOWLEDGEMENTS
We thank R. A. Coutinho for critical reading of the manuscript and suggestions for improvement, F. Dijkstra for providing surveillance data, and A. van der Spek for providing the map showing bulk tank milk-positive farms.
DECLARATION OF INTEREST
None.