Introduction
Hepatitis A virus (HAV), which can result in infections that are self-limited but sometimes serious and occasionally fatal, has the potential to cause community-wide outbreaks among susceptible individuals. Transmission occurs through ingestion of food or water contaminated with HAV or via direct contact with an infected individual, with an ensuing acute viral illness characterised by fever, fatigue, abdominal pain, jaundice and elevated aminotransferase enzymes. After the advent of HAV vaccination in the USA in 1996 and the inclusion of HAV vaccine as a routine vaccination for all children in 2006 [1], incidence declined by more than 95% as of 2015. [2, 3] In the vaccine era, large US HAV outbreaks have been most commonly linked to contaminated imported food products served fresh or uncooked, [Reference Wheeler4–6] although person-to-person outbreaks are an increasingly reported issue as well. [Reference Latash7–Reference Foster and McDonald9].
Correspondingly, HAV infection is uncommon in Hawaii and usually attributed to travellers returning from areas with endemic transmission. During 2005–2015, a median of nine cases was reported annually, statewide. [10, 11] In the week of 20–27 June 2016, the Hawaii Department of Health (HDOH) was notified of four cases of suspect HAV infection. None of the cases had a history of travel to an HAV endemic area and there was no clear link among cases. This report describes the investigation, findings and public health response.
Methods
Epidemiological investigation
Case definition and case finding
HAV infection is an urgently reportable condition in Hawaii (i.e. reportable via telephone by providers and laboratories as soon as a provisional diagnosis is established). Confirmed cases were defined as individuals with acute illness onset with any sign or symptom consistent with acute viral hepatitis (e.g. fever, headache, malaise, anorexia, vomiting, diarrhoea, abdominal pain) plus either jaundice or elevated serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels and positive immunoglobulin M (IgM) antibody to HAV with exposure in Hawaii and no other clear HAV risk factors (e.g. travel to a HAV endemic area).[12] Cases were ascertained through both laboratory and clinician reporting.
On 30 June 2016, HDOH sent a medical advisory to healthcare providers statewide to alert them of the outbreak and the potential for cases among their patients, as well as remind them to report any suspect cases to HDOH. A second advisory was issued on 14 July 2016 to provide guidance on post-exposure prophylaxis (PEP). A Centers for Disease Control and Prevention (CDC) Epidemic Information Exchange alert was posted to inform other jurisdictions of the potential for cases among individuals who travelled to Hawaii before their illness. This posting was updated when subsequently identified exposures impacted other jurisdictions.
Case investigation
Multiple attempts were made to contact all cases reported to HDOH. For those who were unable to be contacted by HDOH, all available medical records were obtained. Cases were interviewed by phone using a standardised questionnaire regarding case demographics, clinical illness history, household and other close contacts, travel, food and other exposures (including specific food-type exposures, restaurants, groceries and potlucks or other gatherings), water source and personal habits (including hobbies and activities, general food and shopping preferences, areas frequented by the case) during the 15–50 days (incubation period range) before illness. Shopper loyalty card numbers were obtained when possible and were used to obtain shopper records from retail grocery chains.
After preliminary investigative data revealed a suspect product, the case questionnaire was revised to assess exposure to that product and to streamline the remainder of the questionnaire. Additionally, cases who had not reported exposure to the suspect product were re-contacted and, using a standard format, were asked specifically about exposure to the suspect product.
Online survey to estimate baseline commercial food consumption behaviours
During the investigation, an online survey (SurveyMonkey Inc., Palo Alto, CA) was created and publicised via news and social media to ascertain respondent home ZIP code, age and locations where food was purchased or eaten in the previous 7 weeks. Locations included national and Hawaii-specific grocery retailers and chain restaurants. Responses were limited to those with a Hawaii state ZIP code. The survey was open for responses from 10 to 16 August 2016. Results were tabulated and respondent ZIP codes mapped on ArcGIS to evaluate coverage throughout the state.
Clinical specimen laboratory testing
Selected case specimens from various points in time and exposure were forwarded to CDC's Hepatitis Reference Laboratory for RNA sequence analysis to characterise the outbreak strain and to compare the sequence to strains present in CDC's sequence database and with sequences from other outbreaks of foodborne hepatitis A. RNA of hepatitis A virus from the specimens was amplified and a 315-nucleotide segment of the VP1–P2B junction was sequenced using published methods. [Reference Nainan13] Cases whose specimens were sequenced were from various points of time during the outbreak and varied in exposure histories and locations.
Data analysis
Epidemiological data were analysed in SAS 9.4 (SAS Institute Inc., Cary, NC) and R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Chi-square tests were used to evaluate differences between dichotomous and categorical sets of variables; P values of ⩽0.05 were considered statistically significant.
Environmental health investigation
Product investigation and trace-back
Distributors and producers were identified for products of interest from restaurants and/or grocers frequently reported by cases to identify commonalities. Recipes were sought to determine component ingredients for products of interest made at retail locations. Invoices were obtained from retailers and restaurants to establish distributors and dates of purchase. With the assistance of the US Food and Drug Administration (FDA), information on distributors and producers outside of the state were obtained. HDOH performed sanitation inspections to observe practices and determine the frequency of product consumption at restaurants and groceries for products of interest.
Product laboratory testing
Once a suspect product was implicated, it was collected for testing at the distributors by HDOH and FDA. Frozen bay scallops from one lot, divided according to the lot's two harvest dates (A and B), were received at FDA Gulf Coast Seafood Laboratory and analysed for HAV utilising a modified protocol of the FDA Bacteriological Analytical Manual chapter 26B. [Reference Williams-Woods, Hartman and Burkhardt14] Briefly, 50 g of individually quick frozen bay scallops (~30) were rinsed with a high pH buffer followed by ultracentrifugation for viral particle concentration. The QIAmp® Viral RNA mini kit and the One-Step RT-polymerase chain reaction (PCR) kit (Qiagen, Valencia, CA) were used for RNA extraction and detection of HAV, respectively. Standard curves were utilised for enumerating HAV genomic copies. [Reference Woods15] Gel electrophoresis of the amplicons from the 5′ UTR of the HAV genome was used to distinguish between wild-type and laboratory controls (unpublished data, FDA). Conventional RT-PCR and big-dye terminal sequencing of the VP1-2B region of the HAV genome was used for sequence characterisation. [Reference Williams-Woods, Gonzalez-Escalona and Burkhardt16, Reference Hutin17]
Outbreak control response
Contact tracing and initial outbreak response
Multiple attempts were made to reach all household contacts of cases by phone to notify them of their potential exposure to HAV and advise them to monitor their health for 50 days after exposure, educate them on signs and symptoms of HAV infection and advise them to speak to their provider regarding PEP consisting of hepatitis A vaccine for people aged 1–40 years and hepatitis A virus-specific IG for people outside of this age range. When cases reported preparation of food for others outside the home (e.g. potluck), attempts were made to reach those individuals as well. When HAV infection was identified in a commercial food handler, the public was alerted via press release so potentially exposed individuals could monitor for symptoms and seek PEP. An outbreak-specific website was created for public education and updates and HDOH staff provided educational outreach and lectures in the community and for healthcare providers.
Results
Epidemiological investigation
Case descriptive epidemiology
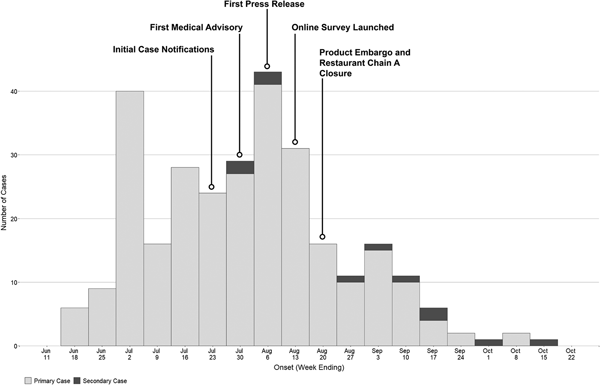
As of 30 December 2016, 292 confirmed hepatitis A cases related to this outbreak were identified by HDOH. The epidemic curve is depicted in Fig. 1. One hundred and seventy-two cases (59%) were male, with a median age of 40 (range 16–80 years). Fewer than 4% of cases (n = 11) were considered possible secondary cases. Seventy-four (25%) were hospitalised and there were two deaths (Table 1). The racial composition of cases was significantly different from 2015 US Census Bureau Data for Hawaii (P value <0.0001); [18] more than two-thirds of cases were Asian, another 21% were Native Hawaiian/Pacific Islander or two or more races and 10% of cases were White (Table 1). Ninety-eight per cent (n = 285) were residents of the state of Hawaii. The most commonly reported symptoms were fatigue (268; 96%), anorexia (258; 93%) and dark urine (255; 92%); jaundice was less common (152; 54%) (Table 1). Of all individuals reported with positive IgM anti-HAV (371) during the outbreak period, 79 were not included as outbreak cases (75 did not meet case criteria for HAV and four had exposures in a country with active HAV transmission). Forty-five (60%) of those not meeting criteria were documented to be asymptomatic via interview and/or review of the medical record.
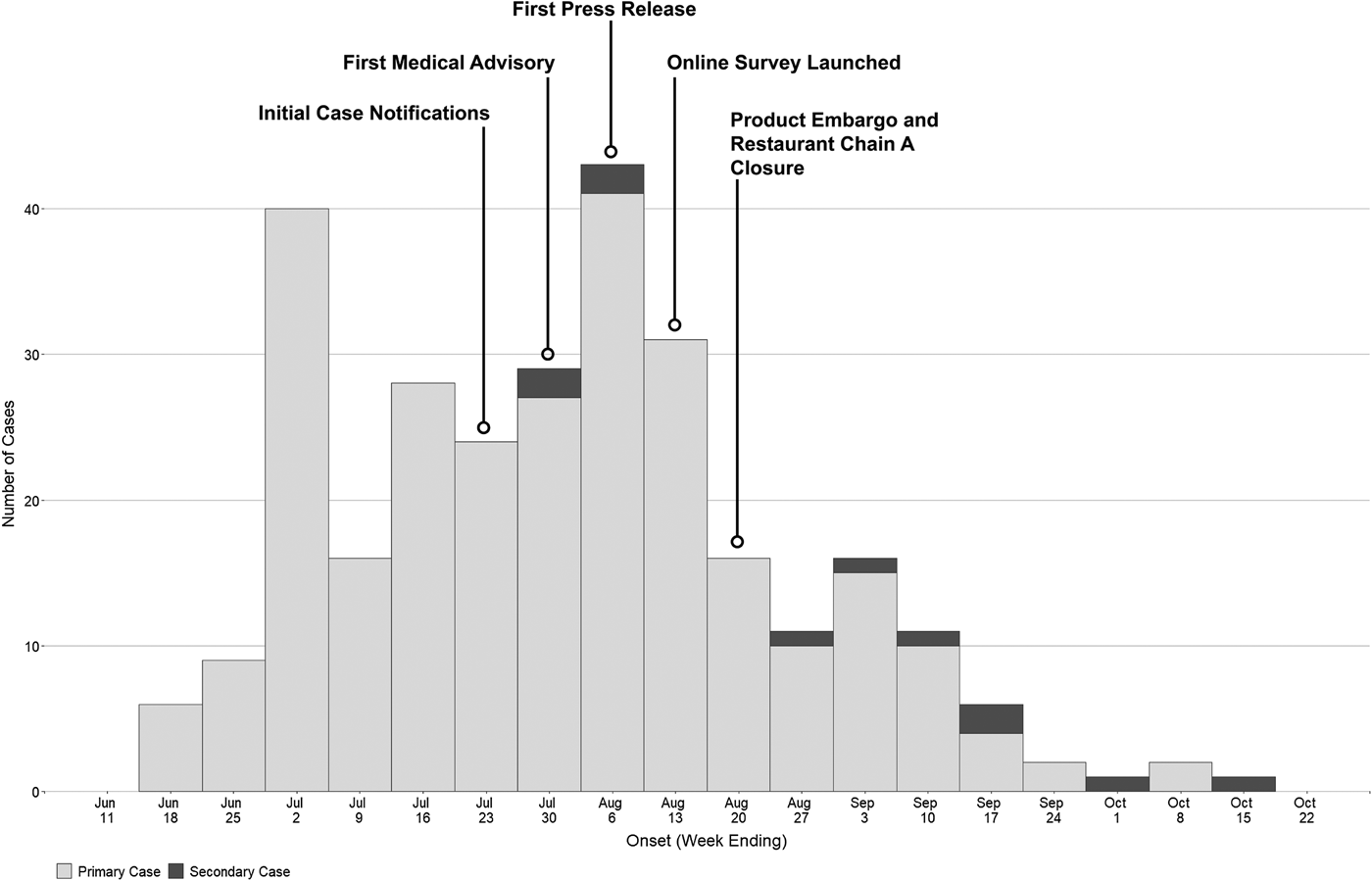
Fig. 1. Epidemic curve of hepatitis A outbreak cases by onset date—Hawaii, June–October 2016.
Table 1. Demographic and clinical characteristics of hepatitis A Outbreak Cases—Hawaii, June–October 2016
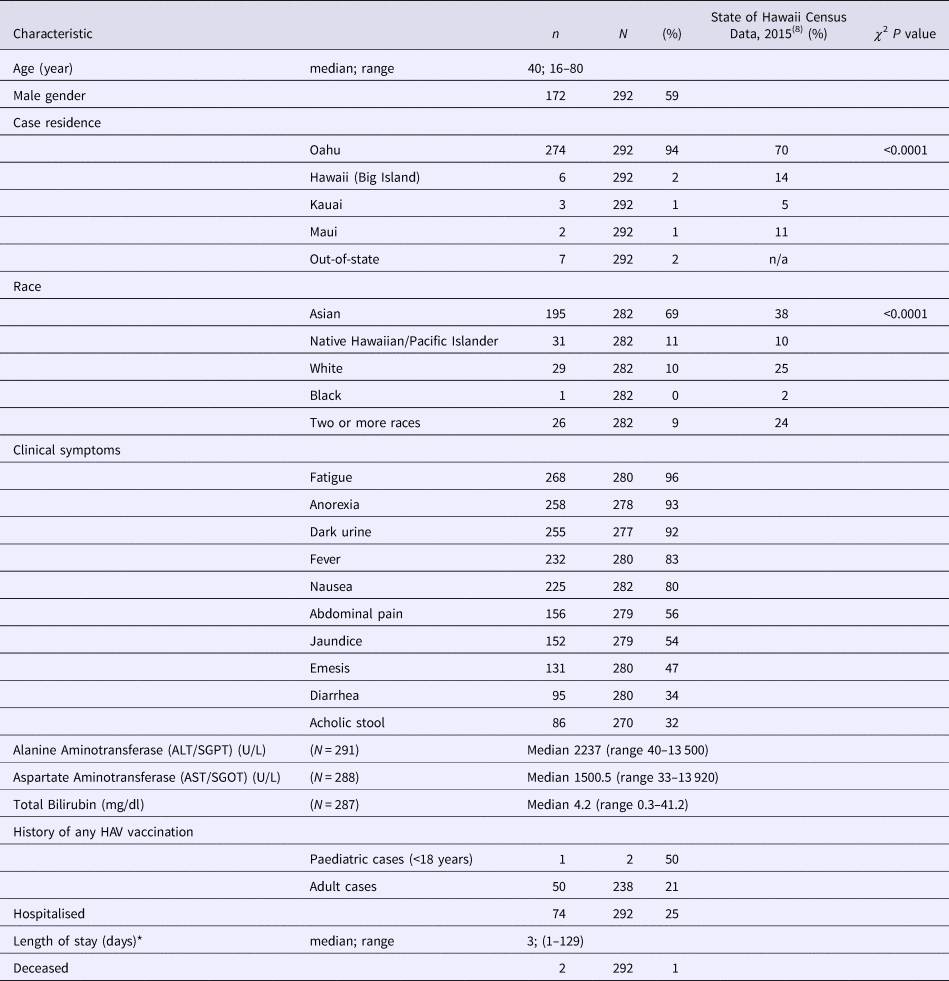
* Length of stay data excludes one individual with prolonged admission for reasons other than hepatitis A infection.
Case investigation
Commonly reported exposures are presented in Table 2. Subset and GIS analyses did not reveal any trends or clustering except that the majority of cases were dispersed across the island of Oahu; no commonalities were identified on review of shopper card data. In primary cases, seafood consumed raw or undercooked was seen in nearly all cases, particularly fish eaten raw, with 95% reporting exposure in the 15–50 days before illness onset. Open text search revealed a mention of ahi (a type of tuna) in 75% of cases.
Table 2. Exposure frequency in hepatitis A outbreak cases—Hawaii, June–August 2016
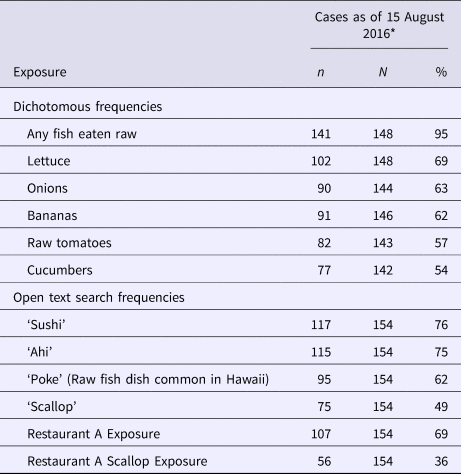
* To present data with minimised recall bias, data are presented only from the initial interviews of the subset of cases interviewed before the first public announcement of a suspect implicated product (15 August 2016).
Preliminary case investigation data on 15 August 2016 revealed that 69% of primary cases reported eating at Oahu or Kauai branches of Restaurant Chain A, a popular chain sushi restaurant with branches located on all islands of the state; 36% reported consuming one of two scallop-containing dishes at Restaurant Chain A. No cases reported eating at Maui or Big Island locations of Restaurant Chain A. No other single restaurant had a reported exposure frequency greater than 36%; exposure to various grocery chains ranged from 12% to 71% (median 48%).
Earlier cases who did not report eating at Restaurant Chain A were re-contacted to specifically inquire about Restaurant Chain A and scallop exposures; new cases were also specifically asked about Restaurant Chain A and scallop exposure. In total, among all cases, 94% reported eating at Oahu or Kauai branches of Restaurant Chain A and 86% reported consuming one of two scallop-containing dishes at Restaurant Chain A. Discussion with Restaurant Chain A headquarters revealed scallop-containing dishes were not one of their top-selling items; actual sales figures were not available.
Online survey to estimate baseline commercial food consumption behaviours
There were 5886 responses to the SurveyMonkey™ online survey; 5841 had Hawaii ZIP codes. The median age of respondents was 46 years (range 3–96). Restricting responses to the island of Oahu (5422 responses), which has the highest density population and had the largest number of cases, 25% of respondents reported exposure to Restaurant Chain A in the preceding 50 days, compared with the 94% of HAV cases who reported exposure to Restaurant Chain A. A similar discordance between survey responses and exposures reported by cases was not seen for any other listed groceries or restaurants on the survey.
Clinical specimen laboratory test results
A total of 68 possible HAV case specimens were sent to CDC for genetic isolation and sequencing. Genotyping at CDC revealed the outbreak strain was genotype 1A. Eighty-five per cent (n = 58) of those sent yielded sequences which matched one another and were unique in the CDC HAV sequencing database. Of the remaining specimens, seven (10%) did not yield genetic material which could be sequenced, two (3%) yielded unrelated sequences and were likely imported disease (13 nucleotide difference) and one (1%) yielded a closely related but distinct sequence (1 nucleotide difference).
Environmental health investigation
Product investigation and trace-back
Examination of distributors of ahi tuna and other commonly reported products or product components (e.g. ingredients for poke) did not reveal any common distributor. Distributors did not routinely maintain records of product lots received or which product lots were sold to which customers. Records that were available were often illegible or incomplete, handwritten, paper records.
No sanitation breaches were identified in Restaurant Chain A locations. Restaurant Chain A locations served the same preparation of raw scallops in two dishes: small (<1 cm diameter) scallops were thawed, then mixed with mayonnaise and tobiko and/or masago (small red-orange fish roe) and served either gunkan style (on top of rice and wrapped in dried seaweed) or seared on top of a shrimp tempura maki (roll). Examination of distributors providing items to Restaurant Chain A revealed a single distributor (Distributor A) whose scallop distribution matched that of the outbreak cases. Scallops were provided to Oahu and Kauai branches of Restaurant Chain A, but not to Maui or Big Island branches. Scallops from a single lot comprising two harvest dates were distributed to Restaurant Chain A during this period but not to any other restaurant or grocer. Scallops from that lot were delivered on three separate occasions to Distributor A from a single out-of-state US importer (Importer A). Importer A reported recently having included a new supplier in the Philippines; the implicated lot was the first lot obtained from that supplier. Trace-back identified the imported scallops were harvested during 2 days in November 2015.
Product testing results
HAV RNA sequences were detected in frozen bay scallops representing both harvest dates of the single lot collected at Distributor A, with levels of contamination averaging four genomic copies per 1.7 grams of scallops. Gel and sequence analysis of the amplicons from Lot A demonstrated that the recovered strain was HAV genotype 1A. In addition, genetic analysis revealed 100% homology between the scallop and clinical strains (unpublished data).
Outbreak response
Based on epidemiologic evidence, on 15 August 2016, HDOH closed Restaurant Chain A restaurants on the islands of Oahu and Kauai and placed all scallops supplied by Distributor A under embargo. Individuals who had consumed food at Oahu and Kauai locations of Restaurant Chain A on or before that date were advised to monitor for symptoms of hepatitis and speak to their providers about PEP if pertinent. Restaurant Chain A locations removed all contaminated product and were thoroughly sanitized. Restaurant Chain A employees were screened for hepatitis A; no cases of HAV infection were found. On 18 August 2016, Importer A voluntarily recalled three lots of frozen scallops. [19]
Data on HAV vaccinations administered and reported to the Hawaii Immunisation Registry (unpublished data, HDOH) from the last week of March through the last week of December 2016 showed a 3.5-fold increase compared with the same period in 2015 (100 634 vaccinations compared with 27 945); 56% (56 122) of 2016 vaccinations occurred during August 2016 and 78% of vaccinees (78 752) during that period of 2016 were adults.
Discussion
We present the second-largest foodborne domestic HAV outbreak in the post-vaccine era. [Reference Wheeler4] Epidemiologic, traceback and laboratory evidence confirmed scallops imported from the Philippines as the source of the outbreak. Our findings highlight the increasing need for improved food safety beyond US borders and the importance of standardised recordkeeping in the food distribution industry to facilitate rapid and accurate trace-back of contaminated food products.
Given increasing global interdependence, there is a pressing need to invest in global food safety. [Reference Doyle20, Reference Wallace and Oria21] In 2013, imported food accounted for 19% of food consumption in the USA compared with 12% in the early 1990s. [22] Fish and shellfish comprise a substantial proportion of imported food, accounting for 17% of all imported food in the USA in 2014. [22] Also, an increasing number of foodborne outbreaks reported in the USA have been associated with imported food. [Reference Gould23] Large foodborne HAV outbreaks have been attributed to green onions imported from Mexico [Reference Wheeler4], pomegranate arils imported from Turkey [Reference Collier5] and strawberries imported from Egypt. [6] The 2011 Food Safety Modernisation Act (FSMA) [24] authorised the use of new tools to improve food safety, including for imported foods. However, implementation of the FSMA is in the process, with roll-out occurring over the span of several years. Furthermore, although the FSMA focuses on the prevention of food contamination and requires processors to keep records of their suppliers and to whom they distribute, there still exists a need for standardised and complete recordkeeping in the food industry to allow for rapid and accurate responses to outbreaks, particularly at the retail and local distribution level. Our investigation was hampered by incomplete information from purchasers and distributors, with substantial delays engendered in inconsistent data and records, if they existed.
It is not clear whether contamination with HAV occurred prior to harvesting the scallops or during processing. Scallops initially seemed an unlikely vehicle given that portions of the scallop involved in filter feeding are not commonly consumed. [Reference Le Guyader25] The presence of HAV in the product strongly suggests contamination with infectious particles at a point before distribution. This is not the first foodborne outbreak in Hawaii related to consumption of imported raw fish or seafood; Hawaii has previously investigated an outbreak of norovirus associated with raw oysters (HDOH, unpublished data) and two outbreaks of Salmonella var Paratyphi B+infections associated with consumption of raw ahi tuna. [Reference Le26, Reference Park27] The same Salmonella serotype was identified in a multistate outbreak also associated with consumption of raw tuna. [28] Although there is inherent risk in the consumption of raw or undercooked seafood, given the popularity of raw seafood consumption, particularly in Hawaii, steps should be taken to assure safe food handling and processing to minimise risk to consumers.
There were several unique aspects to this outbreak. Cases occurred almost exclusively in adults; the two ‘paediatric’ cases were in adolescents, one of whom had no history of vaccination, the other had not completed the vaccination series. Additionally, secondary cases comprised a small proportion (<5%) of the outbreak and while there may be some misclassification of case status (secondary cases may also have had exposure to Restaurant A), there were no paediatric secondary cases identified among the household contacts of cases. A similar trend was seen in the 2013 multistate HAV outbreak associated with pomegranate arils. [Reference Collier5] This is in contrast with findings in HAV-endemic countries or in the pre-vaccination USA. In 2015, Lima et al documented a 34% transmission rate in household contacts of HAV infected individuals in Brazil; [Reference Lima29] pre-vaccination era surveillance data from CDC's Viral Hepatitis Surveillance Program in 1993 reported 4% of cases had personal contact with an HAV-infected patient before illness, 45% of which were household contacts. [30] Routine childhood vaccination against HAV may have had an impact on both outbreak demographics as well as the secondary spread of disease. The 2015 National Immunisation Survey noted 51% of toddlers aged 19–35 months in Hawaii had completed the two-dose series. [Reference Hill31] While lower than the national average (60%), this may underrepresent the number of Hawaii children who ultimately received a second dose as a 2016–2017 estimation of Hawaii kindergarten vaccination rates for HAV was 84%. (unpublished estimate using HDOH data, 2017)
This outbreak also highlights the importance of utilising all available tools and technologies to identify a source of contamination. Baseline rates of population food consumption or restaurant patronage are not always available and approximates from the mainland (e.g. FoodNet Population Survey) [32] may not reflect cultural food preferences in Hawaii. While not truly comparable with our case population and not without limitations, use of an online survey allowed us to obtain a rapid approximation of baseline rates of restaurant or grocery store patronage in Hawaii residents. This approximation helped guide the next steps in the response and investigation and was one of several key pieces of information that helped implicate the contaminated product. While others [Reference Einoder-Moreno33–Reference Yasmin39] have utilised an online survey to rapidly disseminate questionnaires to identified cohorts or a pre-registered body of controls, it has not been previously published as a means to rapidly estimate locality-specific baseline exposure rates. The ability to generate a rapid approximation of local baseline exposures may be critical in determining a suspect source during an outbreak. HAV genotyping and sequencing were also important tools; they distinguished our cases from a concurrent HAV outbreak related to frozen strawberries [6] as well as from isolated imported cases. Relatively few samples have been submitted to CDC's repository of HAV sequences; later outbreaks may benefit further from increased sample submission and ability to identify geographic areas where a particular viral strain has previously been identified.
Our findings have limitations. As exposure information was based on case recollection over a 15–50-day period before illness onset, misclassification of exposure status may have occurred. We advised investigators to use memory aids (receipts, calendars, credit card statements) when possible to improve recollection. Additionally, no formal analytic study was performed to estimate the association between food exposures and case status. Although indicative of overall trends, our rapid online survey of Hawaii restaurant patronage is not directly comparable with our HAV cases. However, the preponderance of investigation data (trace-back, exposure data, product testing) strongly supported our hypothesis, even without a formal epidemiologic study.
In conclusion, our investigation linked consumption of raw scallops imported from the Philippines to a large outbreak of HAV infections and highlights critical gaps in preventing and responding to contamination of an imported food product, a crucial aspect of food safety. Given the global nature of our food supply, quickly identifying and preventing further potential infection are imperative to protecting the public's health. Furthermore, this outbreak serves as a reminder that consuming raw or undercooked seafood continues to carry a risk for foodborne disease. However, our outbreak investigation also presents some assuring signs of the benefits of routine HAV vaccination and promising real-world solutions through innovative electronic and molecular technologies to facilitate rapidly identifying a source.
Author ORCIDs
David I. Johnston http://orcid.org/0000-0002-8866-2118.
Acknowledgements
This work was undertaken with the assistance of all member of the Hepatitis A Outbreak Response Team (DOCD Admin (Nia Barreto, Stacy Richard, Alexandra Ibrahim); DOCD Immunization Branch (Alicia Diem, Paula Doan, Cheryl Donahoe, Emerald Fujioka, Joseph Galatas, Jihae Goo, Mitchell Gutter, Scott Hall, Violet I, Noel Jose, Mariannah Kitandwe, Augustina Manuzak, Marci Nagao, Gail Ogawa, Michael Schweikert, Jocelyn Ungos-Markham, Danielle Vassalotti, James Wasa, Heather Windfield-Smith, Cathy Wu); DOCD Disease Investigation Branch (Myra Ching-Lee, Zeshan Chisty, Arielle Colon, Joe Elm, Hua He, Eleanor Low, Lyle Maesaka, Michelle Nakata, Jed Sasaki, Uyen Truong, Lauren Usagawa, Caron Wilberts, Corliss Yoshiura); DOCD Preparedness Branch (Araceli Barroga, Evelyn Gogue, Jonathan Hilts, Roger Hirata, Judy Kern, Kellen Lovell, Christine Noguchi, Jennifer Roden, Alice Tam, Marjorie Tayao); Public Health Nursing (Ailene Barranco, Derek Bown, Willa Donnelly, Holly Kataoka, Nagisa Kimura, Jean McDermott, Maegan Moleechat, Vivian Murayama, Kristine Omura, Dori Sparlin, Kristin Speltz, Angela Tanioka, Ellen Taoka, Karri Villanueva); STD Branch (Tran Ahn); Maui DHO (Takako Nakaaki); Hawaii Island DHO (Marlena Dixon, Jenny Ushiroda); Kauai DHO (Lisa Gelling); DOH Director's Office (Janice Okubo, Anna Koethe); State Laboratories Division (Rebecca Kanenaka, Roland Lee); Sanitation Branch (Keegan Asato, Chantel Aspuria, David Ching, Greg Edwards, Kathleen Hashimoto, Sandra Hung, Mark Hutchinson, Paul Maeda, Marc Miyamura, Shani Nakanishi, Jennie Negele, Peter Oshiro, Denise Otsuka, Jacqueline Robson, Jill Sakamoto, Alexandra Shibata, Blaine Shishido, Landan Taketa, Erin Villanueva-Miyasaki, Amber Vuong, Lance Wong, Amanda Lowrey, Bill Coleman, Brenda Okazaki, Jennifer Chii, Leona Kealoha, Lisa Yasunaga, Marie Ayson, Mike Okamura, Phyllis Hoomanawanui, Ronald Loo, Sarah Fong, Tristan Asuncion, William Meyers, Lori Nagatoshi, Roger Agullana, Darren Tamekazu, Ricky Oyama, Lisa Otoman-Murayama, Patti Kitkowski, Gerald Takamura, Eric Honda, Lynn Nakasone); Medical Reserve Corps (Susan Fujii, Patricia Fukuda, Joyce Li, Margaret Onaka, Janis Saiki, Roy Yamamoto); DOCD Student Intern (Peggy Su), the CDC Immunization Services Division and FDA (Palmer Orlandi, Toni Morales, Jessica Jones, Williams Burkhardt, Efstathia Papafragkou, Michael Kulka, Rachel Rodriguez, Katja Schilling, Alvin Crosby, Nicole Yuen, Herminio Fancisco, Steven Bloodgood). We received no specific grant from any funding agency, commercial, or non-for-profit sectors. However, HDOH receives critical funding support from CDC through the Epidemiology and Laboratory Capacity and Immunisation grants (6 NU50CK000415-03-09 and 5 NH23IP000721-05-00, respectively), which greatly facilitates our capacity to conduct disease surveillance, investigations and outbreak response.
Conflict of interest
None.