INTRODUCTION
About 25% of humans carry Staphylococcus aureus in the nasal cavities, which act as both its major reservoir, and the most important source for infection [Reference Kluytmans and Wertheim1]. Carriage of S. aureus is influenced by genetic and environmental factors, including cell-wall lipotechoic acid, hormonal status, and antimicrobial activity of nasal secretions [Reference Weidenmaier2]. The pathogenicity of S. aureus has been exacerbated by increased resistance to antibiotics, and methicillin-resistant S. aureus (MRSA), encoded for by the mecA gene, is associated with increased morbidity and mortality compared to methicillin-sensitive S. aureus (MSSA) [Reference Katayama, Ito and Hiramatsu3]. Previously MRSA was largely confined to health-care settings and its acquisition associated with established risk factors [Reference Chambers4]. More recently, it has spread into the community (community-associated MRSA, CA-MRSA), and been implicated in reports of severe infections in otherwise healthy persons [Reference Shopsin5–Reference Hisata7]. Exposure to health care, including surgery, immunosuppression, antibiotic use, and long-term hospitalization, and health-care workers (HCWs) is recognized as a risk factor for hospital-associated MRSA colonization [Reference O'Donoghue and Boost6, Reference Scanvic8, Reference Boyce9], but further work is required to identify reservoirs for CA-MRSA in the community, and their importance as a source of strains imported into the hospital.
Although case reports of human infection or colonization from companion animals [Reference van Duijkeren10–Reference Enoch14] have shown the potential of animals to act as reservoirs for transmission of MRSA [Reference Duquette and Nuttall15], there appear to be no published reports on the prevalence of carriage of S. aureus in owners and their dogs. Reported rates of canine carriage vary widely, but higher levels reported in early studies may be due to the inability to distinguish between S. aureus and S. intermedius, the staphylococcal species most frequently isolated from dogs [Reference Duquette and Nuttall15, Reference Guardabassi, Loeber and Jacobson16]. Although <10% of dogs may carry S. aureus [Reference Duquette and Nuttall15], canine infections with MRSA have been reported [Reference Pak, Han and Shimuzu17, Reference Tomlin18]. Increasing concern about MRSA in the community has led to recommendations for surveillance, including carriage rates in healthy dogs and cats [Reference Duquette and Nuttall15]. S. aureus has been isolated from several sites on dogs, including the anterior nares, skin and anal region, although most studies of carriage sites have focused on S. intermedius [Reference Pinchbeck19, Reference Harvey and Noble20]. It has been suggested that staphylococci are transferred from the nose and mouth of the dog to its coat by grooming and pruritic behaviours, and studies have reported S. aureus in the nares of dogs colonized at other sites [Reference van Duijkeren10, Reference Pinchbeck19]. In case reports of transmission, the dog's nares is the most frequently identified site of colonization when cultures from several sites are performed [Reference van Duijkeren10]. It has been suggested that antibiotic resistance is more frequent in canine isolates of S. aureus [Reference Hoekstra and Paulton21], but this has not been demonstrated in paired dogs and owners.
This study, which is the first to determine the frequency of nasal colonization in healthy dogs and their owners, also investigated the antibiotic resistance patterns of isolates, and strain relatedness between isolates recovered from owners and their dogs. A small subset of stray dogs were examined to compare carriage in dogs with minimal human contact. Risk factors for carriage, including the extent of contact with the dog, were examined. As levels of MRSA carriage in the community remain low [Reference O'Donoghue and Boost6], levels of colonization with both MSSA and MRSA were recorded.
METHODS
Subjects
A cross-sectional study of nasal colonization of dogs and their owners with S. aureus was performed. Adult owners of dogs kept as companion animals and their dogs were recruited at seven veterinary practices over a 6-week period. All owners were invited to participate but, as the study focused on carriage rather than infection, seriously ill dogs, and those with obvious infections were excluded. Most owners were willing for samples to be collected from both themselves and their dogs, with a small number restricting permission to collection only from their dog. The sample size of 800 was based on a carriage level of 25% in adult humans [Reference O'Donoghue and Boost6], and an assumed 25% carriage frequency in dogs belonging to colonized subjects with a 2% error. For comparison of carriage levels in dogs with limited human contact, 30 stray dogs were sampled for nasal colonization. These dogs had been captured by the Society for Prevention of Cruelty to Animals for de-sexing and release.
Specimen collection
Owners were given clear spoken and written instructions, including a diagram, for self-collection of nasal swabs which involved inserting a sterile swab moistened with normal saline, into the nares and gently rotating it to make contact with the nasal septum. As the nostrils of dogs are smaller and more sensitive, veterinarians inserted smaller swabs to a distance of about 0·5–1 cm. All swabs were placed in transport medium and stored at 4°C until culture within 8 h of collection. Owners completed a questionnaire about owner and dog, amount of contact with their dog, and antibiotic use in the dog within the last 3 months.
Laboratory investigation
Swabs were inoculated onto Columbia agar with 5% horse blood (BA), and mannitol salt agar (MSA), and finally placed into cooked meat broth with 5% salt (all media were purchased from Oxoid Ltd, Basingstoke, UK) for enrichment culture, which was subcultured onto BA and MSA after 48 h incubation at 37°C in ambient air. Plates were examined after 48 h and colonies resembling staphylococci were tested with the Murex Staphaurex test (Murex Biotech Ltd, Dartford, UK) for presence of clumping factor. Positives were differentiated from other coagulase-positive staphylococci, including S. intermedius and S. schleiferi, by tube coagulase, pigment production on BA, acetoin production, polymyxin resistance [zone size <10 mm with a 300 U polymyxin B disc (Oxoid)], ortho-nitrophenyl-b-d-galactopyranoside breakdown, and trehalose and mannitol fermentation [Reference Bannerman, Murray, Baron, Jorgensen, Pfaller and Yolken22]. At least five colonies of each isolate were screened for resistance to methicillin by culture on oxacillin-resistance screening agar (ORSAB). Susceptibility to penicillin G (10 U), oxacillin (1 μg), cefoxitin (30 μg), moxalactam (30 μg), tetracycline (30 μg), erythromycin (15 μg), fusidic acid (10 μg), gentamicin (10 μg), vancomycin (30 μg), clindamycin (2 μg), ciprofloxacin (5 μg), rifampicin (5 μg), chloramphenicol (30 μg) and trimethoprim–sulphamethoxazole (1·25/23·75 μg) (Oxoid) was determined by disc diffusion following Clinical and Laboratory Standards Institute guidelines [23], except for fusidic acid, when British Society for Antimicrobial Chemotherapy guidelines were used [Reference Andrews24]. Methicillin resistance was confirmed by detection of mecA using PCR [Reference Ryffel25]. If S. aureus was isolated from both owner and dog and the antimicrobial susceptibility patterns differed by more than two antibiotic susceptibilites, isolates were typed by pulsed-field gel electrophoresis (PFGE) [Reference Prevost, Jaulhac and Piemont26], using SmaI digestion of the bacterial DNA. Chromosomal relatedness was determined by comparison of the banding patterns of the resulting fragments on an agarose gel [Reference Tenover27]. PFGE typing was also performed on MRSA isolates from dogs or their owners attending the same clinic.
Ethical considerations
Ethical approval was obtained from the Human Subjects and the Animal Subjects Ethics Committees of The Hong Kong Polytechnic University confirming that the research was in accordance with the Helsinki Declaration. Owners were given an information sheet about the study and asked to sign consent forms.
Statistical analysis
Prevalence of canine and human carriage was calculated by use of standard equations and included data from all human and dog specimens collected that yielded growth. For determination of association between contact and colonization, only data from dog/owner pairs was considered, each dog and owner being considered as a pair in those cases in which an owner brought more than one dog. The subjects who failed to complete sections of the questionnaire were omitted from these calculations. Differences in proportions of antibiotic resistance were investigated by the χ2 test. Significant associations between categorical variables were determined by Fisher's exact test. Stepwise multivariate logistic regression models were then used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for variables significant in bivariate analysis using the SPSS statistical software package version 15.0 (SPSS Inc., Chicago, IL, USA). Association between age of the dog and colonization was determined by use of a loglinear logit model.
RESULTS
Carriage of S. aureus
A total of 736 owners and 830 owned dogs were sampled for S. aureus carriage. Seventy-five owners (9·2%) were unwilling to be sampled themselves, but gave permission for sampling of their dogs. Most owners (93%) completed the questionnaire, but some did not answer all questions. Eight owners brought more than one dog to the clinic (three owners with five dogs each, one with four dogs, and four having two dogs). S. aureus was isolated from 174 (24%) humans, and 73 (8·8%) dogs (Table 1). In cases of multiple dogs presenting with one owner, no more than one dog was found to be colonized. Isolation frequencies differed between the clinics, but this difference did not reach statistical significance. In 17 cases, both owner and dog were colonized (10% of colonized humans). Based on colony morphology and antibiotic susceptibility pattern, more than one strain of S. aureus was isolated from 12 humans. None of the 30 stray dogs sampled were colonized by S. aureus.
Table 1. Isolation of S. aureus from owners and their dogs at various veterinary clinics
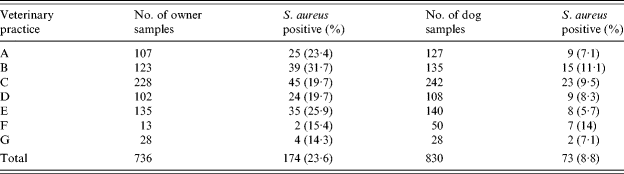
Locations of veterinary clinics: A, Shatin; B, Hong Kong; C, Kowloon; D, Kowloon; E, Hong Kong; F, Sai Kung; G, Tai Wai.
Antibiotic resistance
Almost 90% of isolates were resistant to at least one antibiotic. Canine S. aureus isolates tended to be more resistant than human isolates, with significant differences in frequency of resistance to several antibiotics (Table 2). Only one human isolate was resistant to sulphonamide–trimethoprim, and one dog isolate to rifampicin. Although 15 isolates showed resistance to oxacillin by disc diffusion and growth on ORSAB, PCR did not detect the mecA gene in five of these isolates. Cefoxitin testing identified two of these isolates as MRSA, but moxalactam testing confirmed all as MSSA. Overall, four isolates from humans and six from dogs were confirmed as MRSA. One dog and her owner were both colonized with MRSA.
Table 2. Antibiotic resistance in isolates of S. aureus from humans and dogs
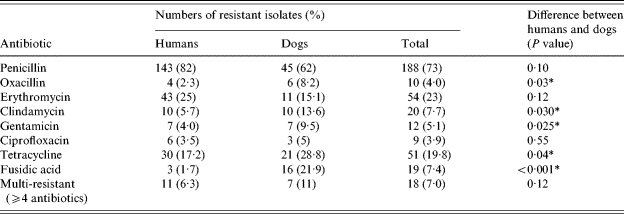
Statistical analysis: χ2 test.
* Significantly more resistance in dog isolates.
Antibiograms of the paired isolates were similar in 11 pairs indicating that both the owner and the dog may be carrying the same strain. Paired isolates with not more than two differences in antibiogram were further investigated for relatedness using PFGE.
Risk factors for S. aureus colonization of owners
Colonization in humans was associated with occupation as a HCW (42% as opposed to 24·9% in non-HCWs) (OR 2·2, 95% CI 1·2, 4·1, P=0·001) and the presence of either a cat or a bird in the household (P=0·001). These factors remained significant after adjustment for other variables using multivariate analysis. Male gender and number of persons in the household were not associated with colonization. Contact with dogs was not found to be associated with increased colonization of owners (Tables 3 and 4).
Table 3. Risk factors for carriage of S. aureus in owners
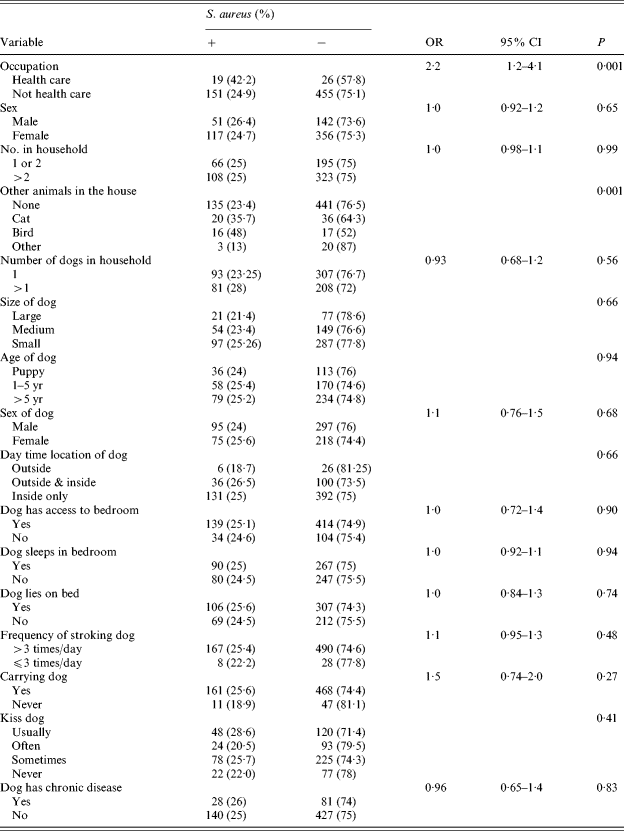
Statistical analysis: Fisher's exact test.
Table 4. Adjusted odds ratios of risk factors for carriage of S. aureus in owners

aOR, Adjusted odds ratio; CI, confidence interval.
Risk factors for S. aureus colonization of dogs
Bivariate analysis showed that nasal colonization was significantly more frequent in female (12%) than male dogs (6%) (P=0·005). Multivariate analysis reduced the level of significance to 0·05. Size of dog, sex of the owner, and use of antibiotics in the dog were not significantly associated with carriage. Dogs of older subjects were rarely colonized, but this did not reach significance due to the low numbers of elderly owners. Dogs in households of ⩽3 people were more likely to be colonized (OR 1·5, adjusted OR 1·6). Bivariate analysis suggested households with 1–3 dogs were less likely to have a colonized dog than households owning more dogs, but this factor was not significant in the multivariate model. Colonization of the owner was not associated with canine colonization, but dogs of HCWs were more likely to be colonized than those of other workers (OR 3·3, 95% CI 1·5–7·3, P=0·002; adjusted OR 2·56, 95% CI 1·1–5·90). Contact with the dog, including stroking, carrying, and kissing or licking the face, did not significantly increase the risk of colonization. Those dogs with access to or sleeping in the bedroom had increased carriage levels, although not reaching significance (P=0·077). Loglinear analysis indicated carriage was associated with the age of the dog, with more colonization in older dogs (10%) in comparison with puppies (5%) and younger dogs (8%) (P=0·01). A similar trend was observed if length of ownership was considered (data not shown) (Tables 5 and 6).
Table 5. Risk factors for carriage of S. aureus in dogs
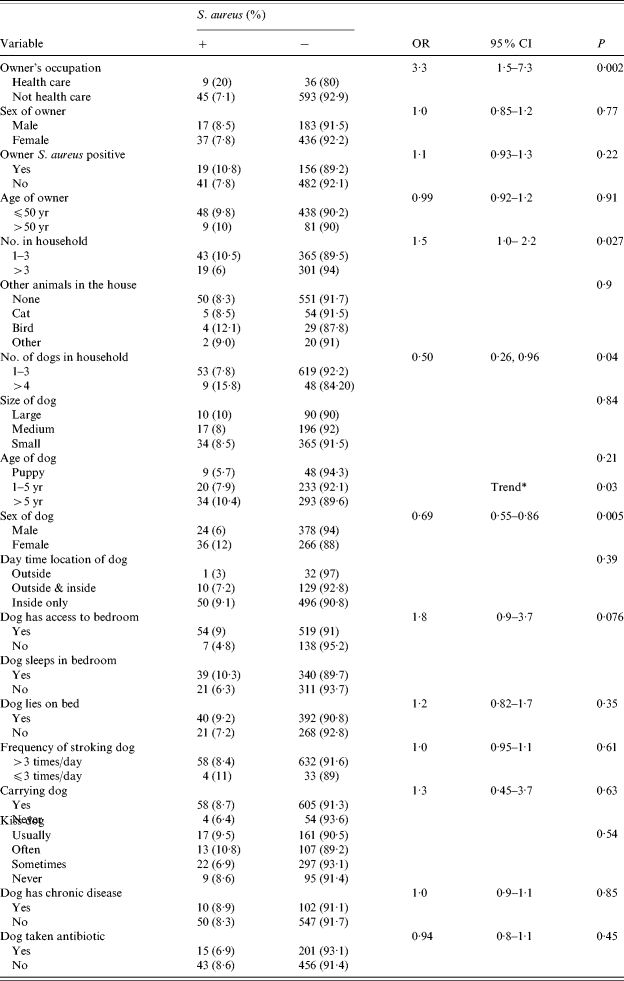
Fisher's exact test.
* Loglinear logit analysis for trend.
Table 6. Adjusted odds ratios of risk factors for carriage of S. aureus in dogs

aOR, Adjusted odds ratio; CI, confidence interval.
Bivariate analysis indicated colonization of the owner, female sex of the dog and access to the bedroom were risk factors for colonization of smaller dogs, but this was not confirmed by the multivariate analysis. Number of people in the household, and an owner employed in health care remained significant risks in this subgroup (P=0·032 and 0·034, respectively) (Tables 7 and 8).
Table 7. Risk factors for carriage of S. aureus in small dogs
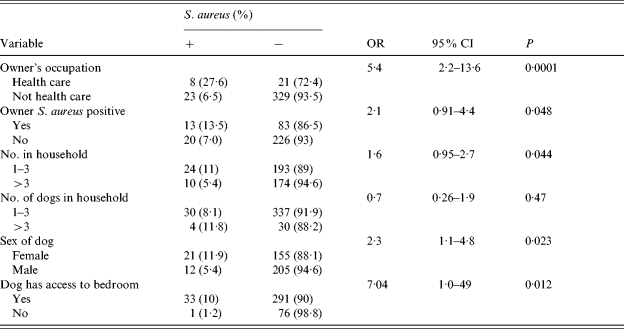
Statistical analysis: Fisher's exact test.
Table 8. Adjusted odds ratios of risk factors for carriage of S. aureus in dogs
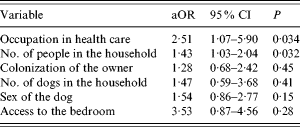
aOR, Adjusted odds ratio; CI, confidence interval.
Characteristics of owner/dog pairs
Although only 6·6% of respondents reported occupation in health care, of the 17 colonized pairs, five owners (29%) were HCWs, and this was significantly associated with dual-colonization (P=0·001). However, only one pair was co-colonized with MRSA. Overall, 11% of HCWs carrying S. aureus had colonized dogs, in contrast with 2·3% of other professionals, 0·6% of clerical workers, 2% of artisans, and 1·8% of students and housewives. Recent canine antibiotic use reduced the likelihood of paired colonization (P=0·022).
PFGE revealed that of 11 pairs with similar antibiograms, six yielded indistinguishable patterns, of which two owners were HCWs. Three of these pairs are shown in the Figure, together with a pair of non-related isolates. Overall, 4·4% of HCWs were colonized with the same strain as their dog whereas only 0·6% of other dog owners carried the same isolate as their pet.

Fig. PFGE of isolates from dogs (D) and their owners (H). Lane 1, K31D; lane 2, K31H; lane 3, K85H; lane 4, K85D; lane5, M113H; lane 6, M113D; lane 7, M203H; lane 8, M203D; lane 9, molecular size ladder. Dogs and humans K31, K85 and M113 appear to be colonized by the same strain, whereas M203 dog and human are colonized by different strains. M203H appears similar to the strain from M113.
Risk factors for MRSA colonization
One of the four MRSA-colonized owners was a HCW, as were the owners of two of the six colonized dogs. Five colonized dogs were female, and five were older dogs (>4 years). Overall, 2·2% of human S. aureus isolates were MRSA, representing a 0·5% colonization rate in the community. In dogs, 8·2% of isolates were MRSA, a 0·7% overall carriage prevalence.
DISCUSSION
The frequency of carriage of S. aureus in owners of 24% was similar to that described previously [Reference Kluytmans and Wertheim1]. The S. aureus nasal colonization level in dogs of 8·8% was similar to the 8·2% carriage rate reported by Biberstein et al. [Reference Biberstein, Jang and Hirsh28], but much lower than the 90% of an early report [Reference Krogh and Kristensen29], which may have suffered from lack of discrimination of S. intermedius. Sampling of multiple sites on the dog may have increased isolation yield, but recent work on carriage of staphylococci in dogs suggests that the same strain tends to colonize the nasal mucosa, the anal region, and the skin of dogs, and that isolation frequencies from the nasal mucosa and anal region are similar, and are higher than from the skin [Reference Hoekstra and Paulton21]. As one aim of our study was to investigate factors influencing transfer of S. aureus between dogs and owners and vice versa, close contact with the facial region would appear to be the most likely, making nasally carried organisms most relevant. Only 10% of the 174 owners with nasal colonization had nasally colonized dogs. Fifty-six non-colonized owners had colonized dogs, suggesting other sources of S. aureus such as another family member, other dogs, food or the environment. However, as carriage in humans may be either persistent or transient [Reference Wertheim30], it is possible that the organism carried by the dog was previously carried transiently by the owner. As a cross-sectional study was performed, colonization could not be categorized in either human subjects or their dogs. The frequency of persistent colonization in dogs is not known, and can only be determined by a large-scale longitudinal study which poses logistical problems. Studies have shown that some dogs, like some humans, never seem to be colonized [Reference Cox31, Reference Rich and Roberts32]. In humans this is thought to reflect the lack of a receptor site on nasal epithelial cells [Reference Wertheim30], and a similar mechanism may also apply in dogs and other animals. The absence of S. aureus in stray dogs suggests that human contact is important, although clearly other factors may affect carriage in stray animals.
The proportion of isolates displaying antibiotic resistance, especially to penicillin and erythromycin was high. The number of isolates exhibiting resistance to several agents was significantly higher among those from dogs than from humans, possibly reflecting higher use of antibiotics in veterinary practice [Reference Guardabassi, Schwarz and Lloyd33]. To our knowledge, this is the first report comparing prevalence of antimicrobial resistance in these two populations of S. aureus. The higher proportion of oxacillin resistance in canine isolates may be due to selection of MRSA by use of first-generation cephalosporins, which are used in the treatment of pyoderma. As the parenteral administration route leads to infrequent use of aminoglycosides in veterinary practice, resistance to these agents is probably co-selected for by use of other antimicrobials. Resistance to two or more antibiotics detected in 54% of canine isolates in our study, was comparable to the 67% multi-resistant clinical S. aureus isolates reported previously [Reference Hoekstra and Paulton21]. High rates of resistance in the present study to both tetracycline (29%) and fusidic acid (22%) seen in canine isolates, may reflect the frequent use of fusidic acid for canine skin and eye infections [Reference Guardabassi, Schwarz and Lloyd33], whilst tetracyline is used as a first-line antibiotic for respiratory infections in veterinary practice. Most antibiotic therapy in companion animals is empirical and pressure from owners may increase antibiotic use [Reference Guardabassi, Schwarz and Lloyd33].
Of isolates phenotypically demonstrating oxacillin resistance, genetic analysis revealed that five did not harbour the mecA gene. Phenotypic false-positives can occur for several reasons, including hyper β-lactamase production, and the results emphasize the importance of confirming phenotypic tests for methicillin resistance with molecular or more sensitive phenotypic methods.
Previous studies have reported isolation of MRSA from infected dogs [Reference O'Mahony34, Reference Loeffler35], and case studies have shown that dogs of both infected patients and colonized subjects may be colonized with MRSA [Reference van Duijkeren10–Reference Simoons-Smit13]. MRSA colonization has also been reported in healthy dogs belonging to healthy MRSA-negative members of veterinary clinic staff [Reference Loeffler35].
The increased risk of nasal colonization of health-care personnel observed in this study has been documented previously [Reference Kluytmans and Wertheim1, Reference Kalmeijer36]. Employment in clinical settings leads to increased risk for MSSA and MRSA colonization. The presence of a cat or a bird was associated with increased risk of S. aureus colonization in human subjects. Cleaning of animal excreta in cat litter trays and bird cages generates dust, possibly increasing respiratory exposure to S. aureus from the faeces of these animals. Unexpectedly, neither the presence of multiple dogs, nor increased numbers of persons in the household, increased the likelihood of carriage in owners. It has generally been assumed that close contact with dogs would increase the likelihood of transmission between human and pet. Most attention has been on the risk of transmission to humans from animals, and people are routinely advised to avoid animals licking their faces. Despite this, owners who admitted frequent close contact with their animals, including kissing the dog and allowing it to lick their face or sleep on their bed, appeared to have no higher risk of colonization with S. aureus than those who did not.
Our finding that nasal colonization with S. aureus was higher in female dogs agrees with data previously published both for S. aureus [Reference Hoekstra and Paulton21] and S. intermedius [Reference Harvey and Noble20], and contrasts with human carriage which is higher in males [Reference Wertheim30]. Hormonal factors may influence colonization, although most female dogs in Hong Kong are de-sexed leading to reduced hormone levels. Behavioural differences between genders may also play a role.
The effect of age on canine colonization, also reported previously [Reference Hoekstra and Paulton21], may be due to immune or other changes in older dogs, but probably reflects increased duration of human exposure.
Increased S. aureus colonization risk for dogs in multi-dog households, although not reaching significance, may be linked to canine social behaviour and has been previously reported for S. intermedius [Reference Harvey and Noble20], as well as to possibly reduced hygiene in these households. Sampling of multiple dogs from one household did not reveal more than one being colonized, but such samples were limited.
The dogs of HCWs were at much higher risk of colonization, with nasal carriage observed in dogs of both currently colonized and non-colonized HCWs. HCWs may carry the organism not only in the nares, but also on their skin and clothing to which the dog is exposed, allowing dogs of transient carriers or even non-carriers to become colonized. Access to the bedroom also appeared to increase risk of colonization as opposed to other living areas. During dressing, undressing, and bed-making contaminated skin scales are shed and are possibly picked up by the dog.
Although the low numbers of colonized dog/owner pairs detected may be affected by transient carriage, it does indicate that transmission between owners and dogs may indeed be low. Of these paired colonizations, again occupation in health care seemed to be the major risk factor. Use of antibiotics in dogs was not a risk factor for colonization, but antibiotic therapy for infection at another site may have also eradicated the colonizing organism. Investigation of the 17 isolate pairs revealed that 11 pairs had indistinguishable antibiograms, but PFGE analysis revealed that five of these pairs were unrelated. In one case, owner and dog were colonized with different strains of MRSA. Thus, only six pairs were indistinguishable by PFGE, although over 90% of owners attending the veterinary clinic with the animal claimed to be the person having most contact with the dog. This again suggests that transfer between owner and dog, or vice versa, does occur, but may be more unusual than indicated by case reports [Reference van Duijkeren10–Reference Simoons-Smit13]. The canine strains may have originated from other family members, but other sources, in particular veterinary practices, may be involved in the transfer of S. aureus to dogs. Although other studies have reported dogs colonized with strains indistinguishable from those recovered from veterinary personnel [Reference O'Mahony34, Reference Loeffler35], in our study PFGE analysis showed that MRSA from dogs attending the same practice differed from each other.
Although MRSA carriage was low, it was noteworthy that of the four owners colonized with MRSA, one was a HCW, whilst two of the six colonized dogs were owned by HCWs. This emphasizes the role HCWs may play in bringing MRSA into the community. The transmission of MRSA to close contacts of HCWs has been previously documented, and previous case reports have implicated dogs of HCWs as reservoirs of MRSA [Reference Manian12, Reference Enoch14]. This study confirmed that dogs of HCWs may be more frequently colonized with either MRSA or MSSA. Of the MRSA-colonized dogs, the preponderance of female dogs is of note, although there is no obvious explanation as to why female gender is a risk factor for colonization.
The primary aim of this study was to investigate the role of dogs as reservoirs for S. aureus, in particular MRSA, following increasing evidence of MRSA colonization in animals [Reference Tomlin18, Reference Rich and Roberts32]. This has been a cause of concern particularly as levels of MRSA have continued to increase in the community, most notably in the United States [Reference Fridkin37] and Australia [Reference Vandenesch38]. Although the prevalence of carriage of CA-MRSA remains low in Hong Kong [Reference O'Donoghue and Boost6], there is still a need for vigilance as infections with community strains of MRSA have recently occurred [Reference Ho39]. In our study, nasal carriage in dogs was assessed, and it was shown that dogs, in particular those owned by HCWs, can act as an important reservoir of the organism.
Whilst this study investigated the association between nasal colonization and close contact with companion animals and their owners in broad terms, its setting conveniently assessed this contact at an extreme level, as Hong Kong is highly urbanized and densely populated, with dogs primarily kept indoors. The majority of the population live in high-rise accommodation and dogs are frequently carried when being taken outside for exercise.
This study has shown that colonization of dogs is primarily associated with occupation of the owner and dog ownership is unlikely to significantly increase the risk of infection in healthy subjects, in particular as kissing and carrying of the dogs was not associated with an increased risk of colonization of either owner or dog. It is recognized that companion animals may serve as a reservoir for infection in immunocompromised humans. As dogs of HCWs are more likely to be colonized, if decolonization of HCWs in outbreak situations is to be performed, consideration should also be given to decolonization of their dogs. Both topical mupiricin or vancomycin have been shown to eliminate carriage in colonized dogs [Reference Manian12], but as topical application of antimicrobial drugs may be impractical, an oral course of rifampcin and either doxycycline or clarithromycin may be employed [Reference van Duijkeren10]. However, care must be exercised in the use of these antimicrobials to prevent increasing risk of resistance and subsequent adverse consequences as these drugs are used in human therapy. The high frequency of resistance in isolates from dogs to antibiotics used for humans is of concern, and indicates a need to re-examine the use of antibiotics in veterinary practice.
This study suggests that transmission is generally from owner to dog, although it may also occur from dog to owner. A large-scale longitudinal study is required to confirm this observation.
ACKNOWLEDGEMENTS
The investigators thank the Research Fund for the Control of Infectious Diseases (grant no. 01030462) for sponsorship of the project. This project would not have been possible without the cooperation and support from the veterinary clinics where sampling was performed and the authors are very grateful for the kindness and help extended by veterinary surgeons and staff at all of the clinics involved: The Ark Veterinary Centre, Sai Ying Pun, Hong Kong; Tai Wai Small Animal & Exotic Hospital, Tai Wai, Shatin; Peace Avenue Veterinary Clinic, Mongkok, Kowloon; Society for the Prevention of Cruelty to Animals (SPCA), Wanchai, Hong Kong; SPCA Kowloon; Sai Kung Animal Hospital, Sai Kung; and Shatin Animal Clinic, Shatin. The authors also thank Miss Sindy Lai, Mr Gilman Siu and Mr Chiu Pak Lung for their technical assistance.
DECLARATION OF INTEREST
None.











