INTRODUCTION
Salmonellosis is one of the most common zoonoses in the world. It was the second most commonly reported human zoonosis in the European Union (EU) in 2008 [1] with a total of 131 468 confirmed cases. The most common sources of human Salmonella infections were eggs, pork and poultry, and Salmonella (S.) Enteritidis and S. Typhimurium were the most frequently reported serovars. However, beef cannot be completely ignored as a source of Salmonella infection in humans even though most member states of EU, including Denmark, reported very low (<1·0%) proportions of beef carcass swab samples testing positive, although some member states reported higher prevalences (up to 7·5%). In Denmark, about 16–36 human cases of salmonellosis were attributed to beef produced in Denmark and about half that number (3–25) to imported beef in 2008. Furthermore, for a large proportion (~40–55%) of salmonellosis cases in humans the source was unknown [2]. Some of these could be from beef or through direct contact with cattle. In 2008, 28 people were recorded as being hospitalized in Denmark with S. Dublin. This serotype is known to be more invasive, difficult to treat and leads to higher mortality in humans than other Salmonella serotypes [Reference Helms3]. This particular serotype is host-adapted to cattle. Thus the source of human cases of S. Dublin is likely to be either beef produced in Denmark, imported beef or direct contact with infected animals and their surroundings.
Specialized veal-producers purchase bull calves from dairy herds around the age of 2 weeks and rear these animals until ready for slaughter. In Denmark, most veal calves are slaughtered before the age of 12 months. They may be infected with Salmonella if bacteria are present in the rearing herd or they may become infected or contaminated during transportation and lairage at the abattoir. In a study from 93 abattoirs in the UK in 2003, seven different Salmonella spp. were found in 36/2553 (1·4%) faecal samples collected from cattle slaughtered at age <30 months. S. Mbandaka was found in 10 (27·8%), S. Typhimurium in 10 (27·8%), S. Dublin in eight (22·2%) and S. Derby in four (11·1%) of the 36 positive samples. The median age of the tested cattle was 24 months [Reference Milnes4]. An older study of apparently healthy veal calves in the UK found 31/720 (4·3%) animals tested infected with Salmonella. Twenty-three of these were S. Dublin. However, only eight (1·1%) of the tested animals had Salmonella in the intestinal contents. The rest were found infected by culture of internal organs, lymph nodes or carcass surfaces [Reference Nazer and Osborne5]. In a review of the importance of S. Dublin in humans in Denmark, Lester et al. [Reference Lester6] concluded that reduction in human S. Dublin infections could be obtained through stricter regulations for the slaughtering of animals from S. Dublin-infected herds, optimal hygiene at abattoirs and increased cooperation between the veterinary and medical professions concerning investigation of routes of infection. It is therefore relevant for the veterinary authorities and the Danish beef industry to learn more about prevalence, serotype distribution and risk factors for Salmonella infection in cattle at slaughter.
There is a lack of studies of risk factors for Salmonella in veal calves. However, suggestions of factors of importance for Salmonella occurrence in cattle generally include hygienic factors in the herds, e.g. flies in pens [Reference Vanselow7], contact with poultry manure or wild bird manure, outdoor calving, herd size and herd expansions [Reference Warnick8]. Hygiene and contacts at markets and in vehicles are also likely to be important risk factors before slaughter [Reference Wray9]. In Danish dairy herds, risk factors for becoming infected in 2003 included herd size, number of purchased cattle from test-positive herds and number of test-positive neighbour herds. Organic herds were less likely to recover than conventional herds indicating that different types of management can influence the occurrence of Salmonella in cattle herds [Reference Nielsen, Warnick and Greiner10]. Off-farm rearing of heifers has been acknowledged as an important risk of infection with multi-drug-resistant Salmonella in US dairy herds [Reference Adhikari11]. One study also reported that for heifers and cows, recent antimicrobial treatment increased the probability of isolating Salmonella from faecal samples [Reference Warnick12]. Moreover, Salmonella has been associated with high calf mortality in dairy herds. This may be due to both direct effects of the infection and underlying management factors [Reference Nielsen13].
The Danish Cattle Federation and the Danish Veterinary and Food Administration initiated an active surveillance programme for S. Dublin in 2002. In short, the programme consists of testing based on regular bulk-tank milk testing of dairy herds and blood sampling mainly at slaughter of non-dairy herds [Reference Warnick14]. All collected samples are tested for antibodies directed against serogroup-D Salmonella antigens. S. Dublin is by far the most important serogroup-D Salmonella type for cattle. Whereas herd classification in the programme is not perfect, it is aimed at classifying herds into groups with low (level 1) and high (level 2) risk of becoming infected with S. Dublin infection, plus a third group (level 3) of herds diagnosed with clinical salmonellosis from S. Dublin. Level 1 herds that purchase animals from level 2 herds become classified as level 2 herds for a period of at least 3 weeks and until new tests from the herd allow it to be promoted to level 1 [Reference Jordan, Nielsen and Warnick15]. This has markedly reduced the movement of animals from level 2 herds to level 1 herds and has contributed to a marked reduction from 26% to 9% national dairy herd-level prevalence from 2002 to 2010. The incidence of human cases of S. Dublin has not decreased proportionally over the same time period. Trade and hygienic slaughter restrictions apply to level 3 herds. However, it is not clear to what extent cattle from levels 1 and 2 pose a risk of introducing Salmonella to the abattoir and whether trade and hygienic slaughter restrictions may be relevant for other herds than those with clinical salmonellosis in order to further improve food safety.
The objectives of this study were (1) to estimate the prevalence of specialized veal production herds that deliver Salmonella to abattoirs in Denmark via shedding animals, (2) to estimate the prevalence of veal bull calves that carry Salmonella bacteria in the colorectal contents at slaughter and determine the concentration of bacteria in Salmonella-positive faecal samples and (3) to determine herd risk factors for Salmonella in veal calves at slaughter. Such risk factors can potentially be used to classify high-risk herds so that special hygienic measures can be taken at transportation, lairage and slaughter to avoid contamination of carcasses.
MATERIALS AND METHODS
Selection of herds
In the study design the aim was to sample 20 bull calves from each of about 80 specialized veal-calf herds from 1 October 2007 until sufficient samples had been collected by sampling a maximum of five animals per delivery from each of these herds. Four cattle abattoirs from three different companies were selected by convenience, and they slaughtered calves from all over the country. Herds were selected based on number of slaughtered bull calves at these four abattoirs in the period 1 September 2006 to 1 September 2007. In total, 200 herds slaughtered more than 100 bull calves aged between 6 and 14 months at these four abattoirs in that period. Out of these 200 herds, 81 herds were randomly selected to participate in the project and sampling was initiated in November 2007. When sampling was stopped in April 2008, 70 herds had calves sampled at the four selected abattoirs and one herd was sampled at a fifth small private abattoir. The last 10 selected herds had either ceased production or changed to delivering calves to an abattoir not included in the study.
Collection of faecal samples
When bull calves between the age of 6 and 14 months from any of the selected herds entered the abattoirs on Mondays to Thursdays, the first five calves in the delivery were marked for sampling. If there were fewer than five animals delivered for slaughter in one day they were all sampled. After removal of the gastrointestinal tract at the slaughter line, faecal samples were collected by cutting into the rectum or colon with a hot-water sterilized knife. About 70 g faecal material was collected aseptically and placed in a container which was marked and stored at 4°C. Samples were sent to the analysing laboratory on the same day or the following morning.
Bacteriological culture method
Faecal samples were all examined at the Regional Northern Laboratory of the Veterinary and Food Administration (Aalborg, Denmark) according to ISO 6579:2002/Amd 1 2007 [16]. Faecal material (25 g) was mixed in 225 ml buffered peptone water (BPW) and left for pre-enrichment at 37°C for 18±2 h. Inoculation of 0·1 ml test material onto modified semi-solid Rappaport–Vassiliadis medium base (MSRV agar) plates was followed by incubation for 48±6 h at 41·5°C. MSRV plates were read after 24±3 h and after 48±6 h. Material from MSRV plates suspected to be positive was inoculated on xylose lysine deoxycholate agar (XLD) (Oxoid CM0469), Salmonella chromogenic agar (SCA) (Oxoid CM1007) and modified Brilliant-Green Phenol-Red lactose sucrose agar (BPLS agar) (Oxoid CM0329) plates and incubated at 37°C for 18–24 h. Isolates suspected to be Salmonella positive at XLD, SCA and BPLS were identified using Salmonella antiserum Poly A-I+Vi and API: ID 32 E. Serotyping and confirmation of positive isolates were conducted at the National Food Institute, Technical University of Denmark (Copenhagen).
All Salmonella-positive faecal samples were cultured by a semi-quantitative method, where the samples prior to pre-enrichment were diluted tenfold with BPW. Five dilutions of each sample were examined separately as described above.
Data for risk factor analyses
The following explanatory factors were assessed in the statistical analyses: purchase patterns, herd size, and calf mortality. Data for the risk factor assessments were collected from the Danish Cattle Database. All data were compiled at herd level. Purchase patterns were evaluated as the total number of animals purchased, number of animals purchased from herds not classified as low-risk herds and number of purchase events from herds not classified as low risk during the period 1 September 2006 to 1 September 2007 (the year before selection of herds for the study). Herd size was defined as the average number of male cattle in the herd during the same period. This number was adjusted for the number of days each animal spent in the herd.
The number of calves that died or were euthanized between ages 1 and 180 days from January 2007 to January 2008, adjusted for the average number of calves in the herds, was used as an estimate of the calf mortality at herd level. The calculation method has been described in detail elsewhere [Reference Nielsen13]. The mortality percentage was transformed for the statistical analyses using the natural logarithm because the distribution was very right-skewed.
Statistical analysis
Apparent herd-level and animal-level prevalence of faecal shedding was calculated directly from the laboratory results and an estimated true animal-level prevalence was calculated by adjusting the apparent prevalence estimates by the estimated sensitivity and specificity of the bacteriological culture test from a previous study [Reference Baggesen17].
The association between the herd probability of delivering animals shedding Salmonella to the abattoir (yes/no) and the explanatory factors described above was tested by logistic analysis using proc genmod in SAS® v. 9.2 (SAS Institute, USA). All explanatory factors were included in a full model and removed by stepwise backwards elimination requiring a significance level of 5% to remain in the final model. After reducing the model to only significant effects, all explanatory factors were then re-introduced one by one in the model to evaluate possible confounding and test for interactions with the main effects. However, total number of animals purchased, number of animals purchased, and number of purchase events from herds not classified as low risk were highly correlated and could therefore not be included in the model simultaneously.
RESULTS
Bacteriological culture results and estimated prevalences
In total, 1296 faecal samples were collected from 71 herds. Due to practical constraints at the abattoirs the number of samples collected from each herd was not easy to control and thus varied from 5 to 40 (mean =18·3, s.d.=6·5) in the final dataset. In total, 20 faecal samples were culture positive for Salmonella spp. The animals testing positive came from 13/71 herds. One herd had four positive samples, one herd had three, two herds had two each and nine herds had one culture-positive sample. All isolates were S. Dublin except one which was S. Typhimurium DT40. No herd had more than a single serotype isolated. It was possible to perform semi-quantitative estimation of Salmonella concentrations in 18 of the S. Dublin-positive samples. Sixteen samples had <1 colony-forming unit (c.f.u.)/g faeces, one had between 1 and 10 c.f.u./g and one had between 100 and 1000 c.f.u./g.
The apparent prevalence of veal herds delivering animals carrying Salmonella infections in the colon or rectum to the abattoirs was 18·3% (95% CI 9·3–27·3). The overall apparent prevalence of culture-positive individual animals across all 71 herds was 1·5% (95% CI 0·9–2·2). In the 12 S. Dublin culture-positive herds the apparent prevalence of Salmonella-shedding animals varied from 4·3% to 20%.
The estimated true prevalence of shedding animals at slaughter was calculated assuming an average test sensitivity of 80% based on a previous study of spiked samples with low concentrations of S. Dublin bacteria [Reference Baggesen17], and a test specificity of 99·5% allowing for a small risk of cross-contamination of the samples at the abattoir or laboratory. The true prevalence estimate of shedding animals at slaughter was 1·3% across all herds, and it varied between 4·8% and 24·5% in the 12 culture-positive herds.
Descriptive analysis of risk factors
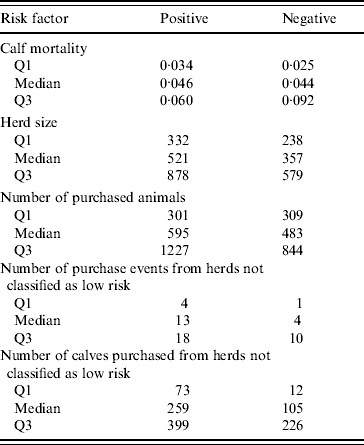
Table 1 provides the distribution of risk factors for each Salmonella-positive and -negative herd.
Table 1. Distribution of risk factors with continuous outcomes [described by 25% (Q1) and 75% (Q3) quartiles] relative to bacteriological Salmonella status of 71 specialized veal herds
Risk factor analyses
One explanatory factor was significantly associated with faecal culture positivity in the logistic analysis, namely the number of times the veal herds purchased animals from herds not classified as low risk in the surveillance programme (intercept=−2·14, parameter estimate=0·068, P=0·03). The predicted association is depicted in Figure 1. None of the other confounders or interactions were significant. However, number of animals purchased from herds not classified as low risk was borderline significant (P=0·06) in a univariable logistic regression model. Herd size was also borderline significant when included in a univariable logistic regression (P=0·06).
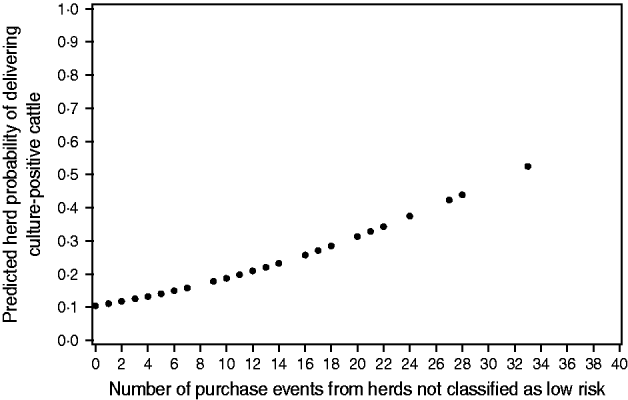
Fig. 1. Predicted probability of veal herds delivering Salmonella-shedding animals to slaughter vs. number of purchase events from high-risk herds during a 1-year period prior to sampling.
DISCUSSION
In this study we estimated the prevalence and concentrations of Salmonella in faecal samples at slaughter in Danish dairy calves reared in specialized beef production herds that delivered more than 100 calves to slaughter per year. These herds are interesting because they contribute the highest load of Salmonella to the abattoirs due to the highest herd prevalence and the high number of animals delivered to slaughter at an early age. We found that at least 18% of the veal herds delivered Salmonella-shedding calves to slaughter in the study period from November 2007 to April 2008. Some herds were probably misclassified as negative due to poor sample size and lack of diagnostic sensitivity of the bacteriological faecal culture test. The concentrations of bacteria in the culture-positive samples were very low which further adds to lack of sensitivity in the culture test. We used a sensitivity of 80% based on a previous study on spiked samples [Reference Baggesen17], but the concentrations may in fact have been lower in this study and other strains may have been involved, so we may have overestimated the sensitivity and thus underestimated the true prevalence of infected animals.
The herds were randomly selected from veal herds delivering more than 100 animals to slaughter per year, and sampling was aimed to be spread out over several deliveries, in order to estimate the true prevalence of animals carrying Salmonella bacteria in their intestines thereby acting as important reservoirs of contamination of carcasses at the abattoirs. The estimate of 1·3% shedding animals corresponds well to the 1·4% found in a similar study from the UK [Reference Milnes4]. However, the majority of the isolates were S. Dublin in our study, whereas in the study by Milnes et al. [Reference Milnes4] only 22% of the isolates were S. Dublin. The overall prevalence might have been higher if we had collected the samples in late summer and early autumn instead of winter and early spring [Reference Davison18, Reference Cummings19].
Calf mortality was not associated with Salmonella shedding at slaughter in our study. This may be because there are other more common reasons for calf mortality in veal calves than Salmonella, including viral diseases such as BRSV and enzootic pneumonia.
Purchase of animals from herds that could not be classified as low risk (level 1) in the Salmonella surveillance programme was found to be associated with isolation of Salmonella from the herds. This is most likely because such herds are often infected with Salmonella and infectious animals are purchased into the veal herd. Similar associations have been found in dairy herds [Reference Nielsen, Warnick and Greiner10, Reference van Schaik20]. This result is important because it provides an option for the control of Salmonella in the cattle industry. If purchase from other than low-risk herds can be limited or stopped – for instance by reducing the number of infected dairy herds or by legislation against such trade, the number of infected veal herds can be reduced. This would be expected to lead to reduced input of infectious animals to the abattoirs.
Although excretion of S. Dublin bacteria at the time of slaughter was generally low with an anticipated low impact on food safety, high shedders will occur at the slaughter line from time to time, but cannot easily be predicted based on register data and controlled per se. The results of this study strongly suggest that Salmonella infection can be controlled in veal herds by avoiding purchase of calves from infected herds. This should probably be supported by management aimed at controlling the spread of Salmonella between calves within the herds.
ACKNOWLEDGEMENTS
The authors thank the Danish Veterinary and Food Administration and the Milk Levy Fund and Cattle Levy Fund for funding the study, and the Danish Cattle Federation for providing data. We also thank the abattoirs involved and the laboratories for helping with the sampling and testing of samples.
DECLARATION OF INTEREST
None.




