INTRODUCTION
Toxoplasma gondii is probably the most common parasitic protozoon in humans in the developed world. Its prevalence is estimated to be more than 30% and varies between 5% and 100% in different countries depending on environmental conditions, hygienic standards and kitchen habits [Reference Tenter, Heckeroth and Weiss1, Reference Pappas, Roussos and Falagas2]. T. gondii is transmitted from an intermediate host, which can be any homeothermic animal, to the definitive host, which can be any species of small or large cats, by predation [Reference Tenter, Heckeroth and Weiss1] and from definitive hosts to new intermediate hosts by ingestion of oocysts, the product of sexual reproduction of T. gondii in the cat's intestine, which are excreted by the millions in the feces of infected cats [Reference Dubey3].
Despite the life cycle of T. gondii being known for a very long time, some aspects of its transmission to humans are still mysterious. For example, in more than two thirds of women that acquired infection during pregnancy, no contact with any known or suspected source of infection was identified [Reference Boyer4, Reference Petersen5]. Many studies showed that contact with cats, touching garden soil or eating raw meat were important epidemiological risk factors [Reference Ferreira6–Reference Cong8], while other studies did not demonstrate their role in transmitting the infection [Reference Minbaeva9, Reference Sharbatkhori10]. A few studies detected some unusual predictors of being T. gondii infected, such as keeping rabbits for meat [Reference Minbaeva9, Reference Sharbatkhori10], having a dog at home [Reference Etheredge11], having mice in a house [Reference de Moura12], eating raw shellfish [Reference Chiang13], being in a close contact with livestock [Reference Markovich14], and having high rate of sexual activity [Reference Flegr, Klapilová and Kaňková15, Reference Alvarado-Esquivel16].
The aim of the present study was to search for independent predictors of T. gondii infection in a Czech and Slovak internet population of 1865 subjects by using a detailed electronic questionnaire for acquiring epidemiological data and a complex model for identifying the most probable risk factors for T. gondii infection. The specific aim of the study was to search for the possible roles of contact with cats and dogs in the transmission of T. gondii to human hosts.
METHODS
Participants and procedure
The participants were invited to participate in the study using a Facebook-based snowball method [Reference Kankova, Flegr and Calda17]. It was done by posting an invitation to participate in ‘an experiment searching for associations between keeping dogs and cats and health status and personality of a subject’ on the wall of the Facebook page ‘Guinea Pigs’ for people willing to take part in diverse evolutionary psychological experiments (www.facebook.com/pokusnikralici). This Facebook community consists of people of various ages, education levels, occupations, and locations. To keep the study blind and avoid a possible bias, no form of the term toxoplasmosis or T. gondii was mentioned during recruitment, not even in the informed consent form. This omission was approved by the Institutional Review Board (IRB). The participants were informed about the general aims of the study on the first page of the questionnaire. They were also provided with the following information: ‘The questionnaire is anonymous and obtained data will be used exclusively for scientific purposes. Your cooperation in the project is voluntary and you can terminate it at any time by closing this web page. Please share the link to this questionnaire with your friends, for example on Facebook’. The share button had been pressed by 967 participants, which resulted in data from 9125 responders in total from 20 August 2014 to 5 November 2016. The entire study and method of acquiring electronic informed content were approved by the IRB of the Faculty of Science, Charles University in Prague (No. 2015/07).
Immunological tests for T. gondii infection
Most of the women and nearly all of men who know their T. gondii-infection status were tested for T. gondii infection during systematic research of behavioral effects of latent T. gondii infection which has been running at the Faculty of Science for 20 years. During this period of time, we tested more than 3000 students of biology. Most of them have been tested within past 5 years (approximately 300 per year). Within the past 3 years we have also tested about 800 other members of the Guinea Pigs community; we suppose that these subjects are overrepresented among participants of the current study. All testing was performed at the National Reference Laboratory for Toxoplasmosis, National Institute of Public Health, Prague. The complement-fixation test (CFT), which determines the overall levels of IgM and IgG antibodies of particular specificity, and Enzyme-Linked Immunosorbent Assays (ELISA) (IgG ELISA: SEVAC, Prague) were used to detect T. gondii infection status of the subjects. ELISA assay cut-point values were established using positive and negative standards according to the manufacturer's instructions. In CFT, the titer of antibodies against T. gondii in sera was measured in dilutions between 1:8 and 1:1024. The subjects with CFT titers between 1:8 and 1:128 were considered T. gondii infected. Only subjects with clearly positive results of CFT or IgG ELISA tests were diagnosed as T. gondii-infected or T. gondii-free.
Electronic questionnaire
The questionnaire was distributed as a Qualtrics survey. The responders used the five-points Likert scale (1, never; 2, minimally (1–2 times in life); 3, rarely; 4, from time to time; 5, very often), to rate their past contacts with six known or suspected risk factors (eating or tasting raw meat, touching soil during gardening, eating root vegetables that were not washed properly, living in countries of lower hygienic standard, drinking water from suspicious sources such as creeks, being at risk of acquiring sexually transmitted diseases (by having sex without a condom with various people). The responders were asked about the size of the settlement where they spent their childhood (1, less than 1000 inhabitants; 2, 1–5 thousands inhabitants; 3, 5–50 thousands inhabitants; 4, 50–100 thousands inhabitants; 5, 100–500 thousands inhabitants; 6, more than 500 thousands inhabitants). The anamnestic questionnaire also contained questions about the intensity and nature of contact with dogs and cats and about animal-related injuries. Participants were asked to rate the intensity of their life-long contact with dogs and cats using the seven-points scale: (1, never; 2, we kept a dog (cat) only in the past and only for a short time; 3, we kept a dog (cat) only in the past but for a long time; 4, we have one dog (cat); 5, we have two dogs (cats); 6, we have three dogs (cats); 7, we have more than three dogs (cats). Using seven-points scale the responders rated how often they stroke a dog (cat) (1, never; 2, maximally once per year; 3, maximally once per month; 4, maximally once per week; 5, maximally once per day; 6, maximally five times a day; 7, more often), whether he/she consider pleasant the smell of a clean dog (cat) fur (1, definitively no; 2, rather no; 3, neither pleasant, nor unpleasant; 4, rather yes; 5, definitively yes; 6, I love the smell of dog (cat) fur). By moving sliders on graphic scales 0–100, the responders expressed their agreement with following four statements: I like dogs (cats), I am not afraid of dogs (cats), I like to touch a dog (cat), A dog (cat) is a member of a family. In a different part of the questionnaire the responders were also asked to rate the intensity of sustained animal-related injuries using the following scale: 1, never; 2, just in play; 3, yes but just to warn me; 4, yes but not seriously; 5, yes with mediate seriousness; 6, yes seriously; 7, yes very seriously, I had to seek for medical help; 8, I was seriously bitten (scratched) by several dogs (cats). Responders were asked to check the variant describing the most serious injury they suffered. Before the analyses, the responses concerning animal-related injuries were recoded to five-points scale 1 (1 or 2), 2 (3), 3 (4), 5 (6 or 7) to get more regular distributions; however, the results of analyses that used the unreduced scale were approximately the same. The participants were also asked whether they are T. gondii infected. They were reminded that T. gondii is ‘a parasite of cats, dangerous especially to pregnant women’. Implicitly, the response ‘I do not know, I am not sure’ was selected. Responders could change this by selecting either ‘No, I was tested by a doctor and the result of my laboratory tests was negative’ or ‘Yes, I was tested by a doctor and I had antibodies against Toxoplasma’. The responders of our questionnaires always had three options: they could complete any questionnaire absolutely anonymously, they could sign the finished questionnaire by a code obtained after anonymous registration, or they could sign the finished questionnaire by a code obtained after non-anonymous registration (see http://pokusnikralici.cz). Some questionnaires were ‘signed’ by <10% of non-anonymously registered subjects (e.g. the questionnaire about sexual behavior); however, the present questionnaire was ‘signed’ by 31% of the participants. When we checked the information about the T. gondii infection status provided in the questionnaire by 3827 participants of our past experiments with corresponding information in our records, we found nearly perfect (99·5%) agreement. The questionnaire also contained many questions concerning health status of responders, a standard Czech version of the Ten Item Personality Inventory (TIPI) [Reference Gosling, Rentfrow and Swann18], Beck Depression Inventory [Reference Beck, Steer and Brown19], Obsessive-Compulsive Inventory – Revised (OCI-R) [Reference Foa20], and Toxo1 questionnaire [Reference Flegr21]; however, only the responses concerning epidemiological risk factors including the contacts with dogs and cats were used in the present epidemiological study. The questionnaire took participants 20–40 min to complete and about 72% of participants who started the study finished the whole questionnaire.
Statistics
Univariate tests and logistic regressions were performed by the statistical software Statistica v. 10.0, while parallel analysis and factor analysis were performed using R software. Significance was at a 5% level (two-sided). Before statistical analysis, I filtered out <1% of data, because it appeared suspicious (too high or too short body height, too low or too high body mass or age, etc.) The associations between ordinal predictors and T. gondii infection were studied by Spearman correlation. This non-parametric test is not sensitive to the presence of outliers in the data set. Spearman tests and Logistic regression tests (Quasi-Newton method, maximum-likelihood loss function and casewise deletion of missing data) were performed for all participants together and also separately for men and women. The optimal number of factors to be extracted (seven) was computed by parallel analysis before the factor analysis (principal axis factoring, direct oblimin normalized rotation, mean substitution of missing data) was performed. The false discovery rate (preset to 0·1) was controlled with the Benjamini–Hochberg procedure [Reference Benjamini and Hochberg22]. In contrast to the simple Bonferroni's correction, this procedure also takes into account the distribution of P values of performed multiple tests. Therefore, the number of significant results after the correction could be higher than before the correction.
RESULTS
Descriptive statistics
Between 20 August 2014 and 5 November 2016, 3106 men (age: 35·8, standard deviation (s.d.) 12·91) and 6019 women (age 33·2, s.d. 12·73) completed the questionnaire. Four hundred and twenty-two (422) men (age: 34·4, s.d. 13·5) and 1443 women (age 33·1, s.d. 11·77), t 6179 = 1·82, P = 0·07 were aware of their T. gondii infection status. T. gondii-infected participants were significantly older than T. gondii-free participants (36·4 vs. 32·2, t 1862 = −6·72, P < 0·00001). Prevalence of T. gondii infection was higher in 1443 women (30·1%, N = 434), than in 422 men (20·1%, N = 85), χ 2 = 16·0, P < 0·0001. For the age structure of the population, see Figure 1 and for frequency and intensity of contacts with potential risk factors see Table 1.

Fig. 1. Prevalence of T. gondii infection in men and women of different age. Numbers at the top of the columns show the prevalences (in %, vertical orientation) and a number of female/male participants.
Table 1. Frequency and intensity of encountering particular risk factors in 1346 Toxoplasma non-infected and 519 Toxoplasma-infected participants

Table shows fraction of participants (in %) who provided particular answers to various infection risk-related questions. The values printed in bold were computed on the basis of more than nine responders. The questions marked with * were responded on the scale 0–100. Here, the numbers 1–10 denote intervals 0–10, 11–20,…,91–100. The column 1 always, except **, shows the fraction of participants exposed to the smallest intensity of contacts with the variable, the values in the furthest-right non-empty column in a row shows fraction of participants exposed to the highest intensity of contacts with particular risk factor. For the size of place of living in childhood (**), the column 1 shows the fraction of participants who spent their childhood in the smallest villages and the column 7 shows the fraction of participants who spent their childhood in the capital Prague. For details concerning coding responses to particular questions see ‘Methods’ section.
Identification of risk factors for acquiring T. gondii infection: univariate analyses
Table 2 shows the associations between toxoplasmosis and 24 ordinal variables describing the intensity of contact with particular risk factors. To decrease an impact of potential outliers, the associations with T. gondii infection were estimated with Spearman's correlation tests, however, the results obtained with parametric tests (logistic regression) were approximately the same (results not shown).
Table 2. Association between T. gondii infection and potential risk factors

Table shows results of Spearman's correlations. Positive (negative) Spearman R denotes positive (negative) association between particular variables. Significant associations (in two-side tests) are printed in bold. P < 0·005 is coded as 0·00. All significant results remained significant after the Benjamini–Hochberg procedure correction for multiple tests (with tolerated false discovery rate = 0·10).
Reduction of dimensionality
A complicated network of relations between animal-related variables and between animal-related variables vs. T. gondii infection probably exists. To reduce the dimensionality of this data for the subsequent analyses, a factor analysis with 17 variables that characterize relations of particular participants with dogs and cats was performed. Seventeen variables were reduced to seven independent factors, which explained 62% of between-individual variability. After oblimin rotation, it became possible to identify the nature of particular factors on the basis of factor loadings; see Table 3.
Table 3. Nine independent variables describing relation of participants to dogs and cats

Table shows factors loadings for particular factors after oblimin rotation. The variables with high positive (low negative) loadings have the highest positive (negative) correlation with a particular factor.
Association between T. gondii infection and various risk factors: multivariate analyses
The association between T. gondii infection and all analyzed risk factors – sex, age and all seven factors obtained with the factor analysis – was studied by logistic regression containing all independent variables in one model. The number of participants older than 50 was relatively low and prevalence of toxoplasmosis decreased after the age of 50 in men and of 54 in women (Fig. 1) suggesting that serological tests provide false negative results in subjects who were infected a rather long time ago. Therefore, only the participants younger than 50 were included into the final analyses; however, the analyses of the whole set gave very similar results. The results showed that independent risk factors for having anamnestic antibody titers to T. gondii were spending one's childhood in small communities, past direct contacts with garden soil, having consumed unwashed root vegetables, and having suffered a cat-related injury (Table 4). The results were similar when men and women were analyzed separately or when sex–age interaction was included into the model. Due to the lower number of men in our sample (422 vs. 1443), all effects except for the effect of age were non-significant in men. However, the strength of this association estimated on the basis of odds ratio (OR) was sometimes higher in men than in women (cat-related injury: OR = 3·88 vs. 1·93; eating unwashed vegetable OR = 3·64 vs. OR = 1·46). The seroprevalence of T. gondii infection increased with age in women up to age 50–54 and decreased after age of 54. In men, this seroprevalence increased only up to age of 39, stayed stable until age of 49, and decreased after age of 49; see Figure 1.
Table 4. Association between T. gondii infection and potential risk factors
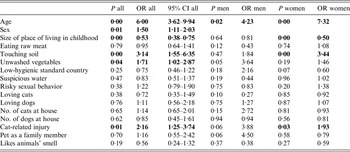
Associations were measured with Logistic regression. Men are coded as 1s, women 2s; therefore OR > 1 reflects the higher probability of being Toxoplasma-infected in women. Significant range Odd Ratios (OR) (in two-side tests) are printed in bold. P value 0·00 means P < 0·005.
DISCUSSION
Analysis performed on the large sample of a population showed that having contact with garden soil, eating improperly washed root vegetables, and being injured by a cat were the most important predictors of the T. gondii infection in our population. The probability of being infected with T. gondii was higher in women than in men and in participants who spent their childhood in small communities. This probability increased with the age of the subject. Surprisingly, the prevalence, which was increasing up to the age 39 in men and up to age 54 in women, generally began showing a decrease when those ages were reached.
The important role of contact with garden soil and consuming unwashed root vegetables is in agreement with our knowledge of the life cycle of T. gondii and the ethology of its definitive host – a cat [Reference Dubey, Janovy and Esch23]. After primo-infection a cat may excrete millions of T. gondii oocysts. Domestic cats living around and in human communities defecate near houses, often in gardens. The oocysts survive and remain infectious in humid soil for years; therefore, the soil in garden patches near houses is often heavily contaminated with oocysts [Reference Gotteland24, Reference Torrey and Yolken25].
The relationship between T. gondii infection and various aspects of pet dogs and cats was in the center of the interest through the present study. It was suggested earlier that a cat as well as a dog can be the proximal source of infection [Reference Etheredge11], that pets could be just an indication of a more rustic style of life [Reference Flegr, Hrdá and Tachezy26] and that the animal-related injuries, especially cat bites, can be a source of T. gondii infection [Reference Hanauer, Ramakrishnan and Seyfried27, Reference Flegr and Hodny28]. It has been also published that, in a double blind experiment, T. gondii-infected male students consider a smell of highly diluted cat urine more pleasant than T. gondii-free control subjects [Reference Flegr29]. Therefore, it can be speculated that infected persons are more enamored with cats and own cats more often than T. gondii-free persons. The results of the complex analysis showed that neither the number of cats nor dogs in the house nor love of cats or dogs had a significant impact on the risk of being T. gondii infected; however, cat-related injuries were found to be predictive of such an infection.
It can be hypothesized that T. gondii parasites could be directly transmitted from the body of a cat to the body of a human by biting or scratching (nearly all participants bitten by a cat were also scratched by a cat). Another hypothetical way of acquiring infection is the contamination of scars with oocysts contained in soil on the cat's claws, paws and other parts of the pets’ bodies; however, no non-oral route of infection by T. gondii oocysts has been described. If this latter possibility holds, then even non-infected cats could transmit T. gondii to a human host if they defecate or just rest at garden where other cats have defecated. Pregnant women are commonly urged not to change cat litter boxes. The present results suggest that a better recommendation is for pregnant women to entirely limit interaction with cats.
The present results confirmed that spending one's childhood in small communities increases the risk of T. gondii infection even when other associated variables like contact with cats and garden soil as well as eating unwashed root vegetables are controlled for. This means that other unknown risk factors associated with living in small communities probably also exist. A study performed on a population of 3250 soldiers showed that the strongest predictor of T. gondii seropositivity was keeping rabbits for meat (OR 1·45, 95% CI 1·22–1·77) [Reference Kolbeková30]. In future studies, this factor should be monitored and included into statistical models.
On the basis of several independent lines of indirect evidence, the existence of a sexual route of transmission of T. gondii infection has been suggested [Reference Flegr, Klapilová and Kaňková15, Reference Alvarado-Esquivel16]. It has been shown that the prevalence of T. gondii infection in particular countries correlates with the incidence of sexually transmitted diseases [Reference Flegr31]. Seemingly, the present results, namely the positive association between T. gondii infection and risky sexual behavior observed in univariate analyses, provided further support for this hypothesis. However, the results of logistic analysis of the complex model did not support the existence of this association. Because of high clinical importance of this potential route of infection (risk of congenital toxoplasmosis) the problem of the sexual transmission of T. gondii infection deserves more attention in future studies.
Neither eating raw meat nor drinking potentially contaminated water was significantly associated with being T. gondii infected. This contrasted with some earlier results and with our knowledge of the life cycle of T. gondii. The most frequent source of raw meat in Czech cuisine is steak Tartar, the minced raw beef with egg and spice. However, the prevalence of T. gondii infection in cattle from large farms is low, the number and viability of tissue cysts in beef is very low and steak Tartar is prepared from frozen meat [Reference Dubey, Janovy and Esch23]. It is highly probable that all T. gondii bradyzoites are killed in frozen and possibly even cooled meat. The growing frequency of consumption of frozen and cooled meat can be, in fact, a reason for the systematic decrease of the prevalence of T. gondii infection in the developed world, including Czechia, during past 20 years [Reference Pappas, Roussos and Falagas2].
A non-significant negative association between T. gondii infection and drinking suspicious water as well as visiting low hygienic standard countries (nearly significant in women) can be the side-effect of a changed personality profile of infected subjects, which is usually considered to be a product of manipulative activity of the parasite. T. gondii is known to increase the concentration of neurotransmitter dopamine in the brain tissue of infected animals [Reference Gatkowska32, Reference Prandovszky33] (but see also [Reference Wang34]), including humans, and increased level of this neurotransmitter is associated with decreased personality factor novelty seeking [Reference Cloninger and Silk35, Reference Wiesbeck36]. The decreased level of novelty seeking monitored with Cloninger TCI questionnaire [Reference Cloninger37] was observed in both men and women infected with T. gondii [Reference Flegr38, Reference Skallová39]. It is highly probable that subjects with low tendency to seek for new stimuli will consume exotic meals such as steak Tartar less often, and possibly also less often drink potentially contaminated water, e.g. water from creeks.
This study confirmed earlier results concerning non-monotonic change in the prevalence of T. gondii infection with age in the Czech male subjects [Reference Kolbeková30]. In the current study, the prevalence of T. gondii infection in men achieved a maximum of 29·7% at ages 35–39, while a similar study performed on the population of 3250 male soldiers showed a maximum of 35% at ages 30–35. Because the first study was performed 10–15 years earlier than the current study, the observed decrease of prevalence of T. gondii infection after age 39 cannot be the effect of cohort (otherwise it should be shifted to age 45–50). On the basis of experience of reactivation of toxoplasmosis in immunosuppressed patients, including AIDS patients, it is widely believed that dormant stages of the parasite survive in the body of infected subjects, especially in their brains, for their whole lives. However, results of longitudinal serological studies as well as case studies and anecdotal observations show that the concentration of anti-T. gondii antibodies irregularly decreases with age of infected subjects, and that some infected subjects could turn seronegative in older age [Reference Kodym40]. On the basis of a permutation test for contaminated data, the fraction of seronegative subjects who are definitively T. gondii-infected was estimated 5%–10% among subjects age 20–40 [Reference Flegr and Havlíček41, Reference Flegr, Hrdá and Kodym42]. In the higher age strata, the fraction of such subjects is probably even higher. It can be speculated that the rate of acquiring new T. gondii infection slows down as subjects become older, and in the age ranges of 35–49 in men and 50–54 in women becomes equal with rate of positivity-to-negativity seroconversions caused by the natural decrease of concentration of anti-T. gondii antibodies. If this model is correct, then the prevalence of T. gondii infection is in fact much higher than the current serological surveys show. It must be reminded that except for the studies performed on relatively young and age-homogenous populations of subjects, such as pregnant women and soldiers, the earlier epidemiological studies were performed mostly on mixed-gender populations with a rather low number of participants older than 50 years. The opposite trends (decrease vs. increase) of change in the prevalence of T. gondii infection in 35–54-year-old men and women, together with a usually rather low number of participants in higher age strata in earlier epidemiological surveys; see for example [Reference Jones, Kruszon-Moran and Wilson43], might explain why this phenomenon – i.e., the decrease of prevalence of T. gondii infection in men older than 35 and women older than 54 – has escaped the attention of other researchers and why most of the previous studies report a stagnation of prevalence of T. gondii infection after the age of 30. The exception was a large study of Kodym et al. [Reference Kodym44] (N = 3431). This study included nearly 500 participants in each 10-years strata and it showed the decrease of prevalence of T. gondii infection in men older than 59 and in women older than 49.
Limits of the present study
A major limit of the present study is the fact that its participants provided information about their T. gondii infection status themselves. At least some of them probably provided incorrect information and some of them (only the T. gondii-free) may have provided obsolete information because they acquired the infection only after their test for anti-T. gondii antibodies had been done. It could be advocated that at least some of old individuals reported the historic status rather than the current status. However, even in such a case the prevalence in older age strata would be either same or higher than in younger age strata. Moreover, the decreased prevalence of toxoplasmosis in older age strata has been reported also in two previous studies in which the participants were directly serologically examined for the presence of specific antibodies [Reference Jones, Kruszon-Moran and Wilson43, Reference Kodym44]. It must be also stressed that stochastic error could only cause false negative results, such as the failure to detect some weak risk factors, but not false positive results, such as identification of an actually non-existent risk factor [Reference Flegr45]. There is no indication pointing to the existence of a systematic bias in the present data (for example, for higher probability of reporting positive toxoplasmosis status by cat keepers). Our independent analysis showed nearly perfect (99·5%) agreement between toxoplasmosis status reported by 3827 subjects during their electronic registration to Guinea Pigs community and toxoplasmosis status obtained in our serological tests. Similarly, we observed nearly perfect agreement (99·2%) in 393 responders who signed their questionnaire in the present study and also reported their toxoplasmosis status during registration to the Guinea Pigs community. Moreover, the only animal-related variables positively associated with Toxoplasma infection were animal-related injuries. These variables are not considered to be toxoplasmosis risk factors by most parasitologists and laymen. In the context of our results on observed rapid decrease of seroprevalence of T. gondii infection in higher age strata and presumed frequent positivity-to-negativity seroconversion in older subjects, it can be argued that the epidemiological studies based on the reported results of passed serological tests could be actually more precise and sensitive than the cross-sectional studies, which rely on the serological examination of the current seropositivity/seronegativity status of participants.
Our systematic testing of subjects for T. gondii infection started about 25 years ago. Therefore, only 87 participants of the present study were older than 60 years. Therefore, the estimated prevalence of T. gondii infection in this age category could be relatively imprecise.
The current study involved mostly healthy people with the so-called latent toxoplasmosis, i.e. the form of the T. gondii infection characterized by the presence of non-sterile immunity against T. gondii and no clinical signs of the disease. It is possible that different sets of risk factors exist for this asymptomatic form of the infection and for the acute and chronical forms of toxoplasmosis. Theoretically, a more serious form of toxoplasmosis could be associated with the infection of humans with tissue cysts than with the oocysts because the tissue cysts are primarily destined for the infection of definitive host, a cat. Many predation transmitted-parasites harm their intermediate hosts, but not their longevous definitive host, which often serve them mainly as a meeting point for sexual partners and as reservoirs and vehicles of gene flow across space and time.
The present study was performed on a sample of internet users, very often previous students of biology at the Faculty of Science, Charles University. It is not clear which results of the study could be generalized to the standard Czech population.
CONCLUSIONS
The present study identified new risk factor for the acquisition of T. gondii infection for a modern population, namely cat-related injuries. The most important result of the present study is the observed decrease of seroprevalence of T. gondii infection, which starts around the age of 50 in men and age 55 in women. Experience with reactivation of toxoplasmosis in the infected immunosuppressed patients shows that T. gondii survives in immunoprivileged organs of human hosts until end of life [Reference Flegr46]. Therefore, the existence of such a decrease can be explained either by increased mortality rate of T. gondii-infected subjects [Reference Flegr31, Reference Flegr and Escudero47] or by frequent positivity-to-negativity seroconversions in infected subjects. If this is true, then either the prevalence of T. gondii infection or its potential impacts on public health could be much larger than generally believed.
ACKNOWLEDGEMENTS
The work was supported by project UNCE 204004 (Charles University in Prague) and the Czech Science Foundation (Grant No. P407/16/20958). I would like to thank to Raymon Gongora, Gary Hawkins, Charlie Lotterman and Lenka Příplatová for their help with final version of the paper.
DECLARATION OF INTERESTS
None.
ETHICAL STANDARDS
The author asserts that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








