Introduction
Despite the intense efforts in preventing HIV-1 transmission, more than 2 million new infections were reported in 2014 worldwide [1]. However, thanks to the advances in care of people living with HIV-1, a drastic decrease in HIV-related morbidity and mortality was reported [Reference Rodger2]. Retention in care of HIV-infected patients and adherence to the prescribed antiretroviral regimen are pivotal for obtaining virological suppression with the resulting long-term health benefits and the reduction in the risk of infection transmission, leading to a possible containment of HIV spread [Reference Antinori3–Reference Chan5]. The continuum of care moves from HIV testing to linkage to care, retention in care, engagement in care with the prescription of combined antiretroviral therapy (cART) until achieving viral suppression and maintenance of status [Reference Gardner6]. Monitoring of continuum of care is essential for maintaining virological success: it can be considered an important indicator of provided assistance quality level. Data from United States highlighted that approximately 77% of people diagnosed with HIV were linked to care, and 51% were retained in ongoing care; only an estimated 28% of all HIV-infected persons in the United States have a suppressed HIV-RNA [7]. In Italy, an estimation of continuum of care was developed in 2014 [Reference Mammone8], extrapolating data from National Surveillance System for HIV and from ICONA cohort (Italian Cohort of Antiretroviral-Naive Patients) [Reference Raimondo9, 10]. An estimated 134 000 persons were living with HIV in Italy of whom 89% had been diagnosed [Reference Mammone11]. Patient retained in ongoing care are approximately 102 000 [Reference Zona12]. Based on Italian cohort studies [10], cART was prescribed for an estimated 83% of patients linked in HIV care (74% of people living with HIV), and viral suppression (<50 copies/ml) was achieved in 87% of treated patients, equal to 52% of HIV-infected Italian patients (69 680 of 134 000). It is evident that the impact of cART on HIV infection-related mortality and morbidity is only evaluable in a portion of the targeted population. Although national and international public health institutions emphasise the importance of population HIV testing and therapy as prevention, intermediate steps of the cascade of care, and in particular the retention in care, are increasingly attractive tools in the fight against HIV infection [Reference Mugavero13, Reference Nachega14]. Notably, failed retention in care, with the resulting delayed start of cART, is associated with a worse outcome including virological failure and increased mortality [Reference Mugavero15–Reference Ulett17]; nevertheless, few studies have payed attention to predictors associated with this endpoint, particularly in industrialised countries. The aim of this study is to evaluate the rate of retention in care for HIV-infected patients in a large University and Community Hospital, in Italy, and to identify predictors associated with failed retention in ongoing care. Virological success and predictors of failed cART efficacy are also evaluated as secondary endpoint.
Methods
The present study is a prospective, observational, cohort study involving all new HIV-1-infected patients diagnosed at the Clinic of Infectious Disease and at the Clinic of Immunology and Internal Medicine of Ospedale Policlinico San Martino of Genoa, a large city in the North West of Italy with nearly 600 000 inhabitants. Data collection began the 1st of January 2008 and ended the 31st of December 2014; all patients were consecutively enrolled in the study. All data were collected on the MedInfo online platform enclosed in the Ligurian HIV Network. The MedInfo platform (www.reteligurehiv.it) is an online database that allows anonymous and automatic data collection (laboratory test results and clinical information) for HIV-infected patients in the Ligurian region. Data are encrypted to ensure privacy but easily available to physician. The online database contains more than 2500 patients and allows the execution of multicentre clinical trials [Reference Fraccaro18]. Key inclusion criteria were: age >18 years and documented HIV-1 infection. All participants were evaluated at baseline and demographic parameters (age, sex and nationality), plasma HIV-1-RNA levels, nadir lymphocyte T CD4+ (CD4+) cell count, HBV, HCV co-infection and CDC stage. cART regimens were registered at baseline and at scheduled follow-ups until 1 July 2015. The loss to follow-up was defined by the lack of outpatient physical examination and/or cART withdrawal and/or absence of laboratory tests for more than 6 consecutive months. Patients who died or moved during the study were considered lost to follow-up as well. In all patients actively on cART, virological suppression was defined as HIV-RNA <50 copies/ml after 6 months of therapy. The study was conducted according to the Declaration of Helsinki; on 21 October 2014, the protocol was reviewed and approved by the Liguria regional institutional review board/independent ethics committee (P.R. 353REG2014).
Statistical analysis
For clinical and demographic data, descriptive statistics were summarised in terms of medians with first and third quartiles (1st–3rd quarter) or in terms of absolute frequencies (percentage). Comparisons of disease characteristics between patient groups were performed by the χ 2 test (or by Fisher's exact test in case of expected frequencies <5) for categorical variables. For continuous variables, the comparison between patient groups was performed by Student's t test if reasonably normally distributed, otherwise Wilcoxon test was used. A P value of <0.05 was considered as statistically significant. The statistical package used was SAS 9.3 (Institute Inc., Cary, NC, USA).
Stepwise multivariate logistic regression analysis was performed, with loss to follow-up or efficacy after therapy as the dependent variable, and all variables with a significant univariate P-value as covariates. The final model was arrived at by means of a step-down procedure, based on the likelihood ratio test.
Results
In this study, 269 HIV-infected patients followed at Ospedale Policlinico San Martino of Genoa were enrolled and linked in care. Mean age was 46.1 years (38.6–54.1). Male were 197 (73%), Italian patients were 219 (81%). Mean length of disease was 5.1 years. One hundred and fifty-eight patients (59%) had a baseline lymphocyte T CD4+ cell count >200/mmc. Co-infection with hepatitis C and B virus was present in 32 (13%) and 7 (3%) patients, respectively. The rate of retention in care was 78.4% (211/269) after 6 months of follow-up. Among the patients who failed maintenance in care, 14 (24%) died and 10 (17.2%) moved to other medical centres. cART was overall prescribed for 257 patients (95%) and for 209 among retained patients (99%). Out of these subjects, 204 had HIV-RNA <50 copies/ml. The rate of virological success, considering the entire population of enrolled patients, was 75%. The cascade of continuum of care of HIV-infected patients is illustrated in Figure 1. In the univariate analysis being foreign-born patients, compared with Italian-native patients was statistically significant for failed retention in care as well as HBV co-infection, HIV-RNA >50 copies/ml at the last performed follow-up evaluation, absence of cART and low CD4+ cell count at the baseline. Demographic parameters of the population in the study and differences among patients linked to care and lost at the follow-up are shown in Table 1.
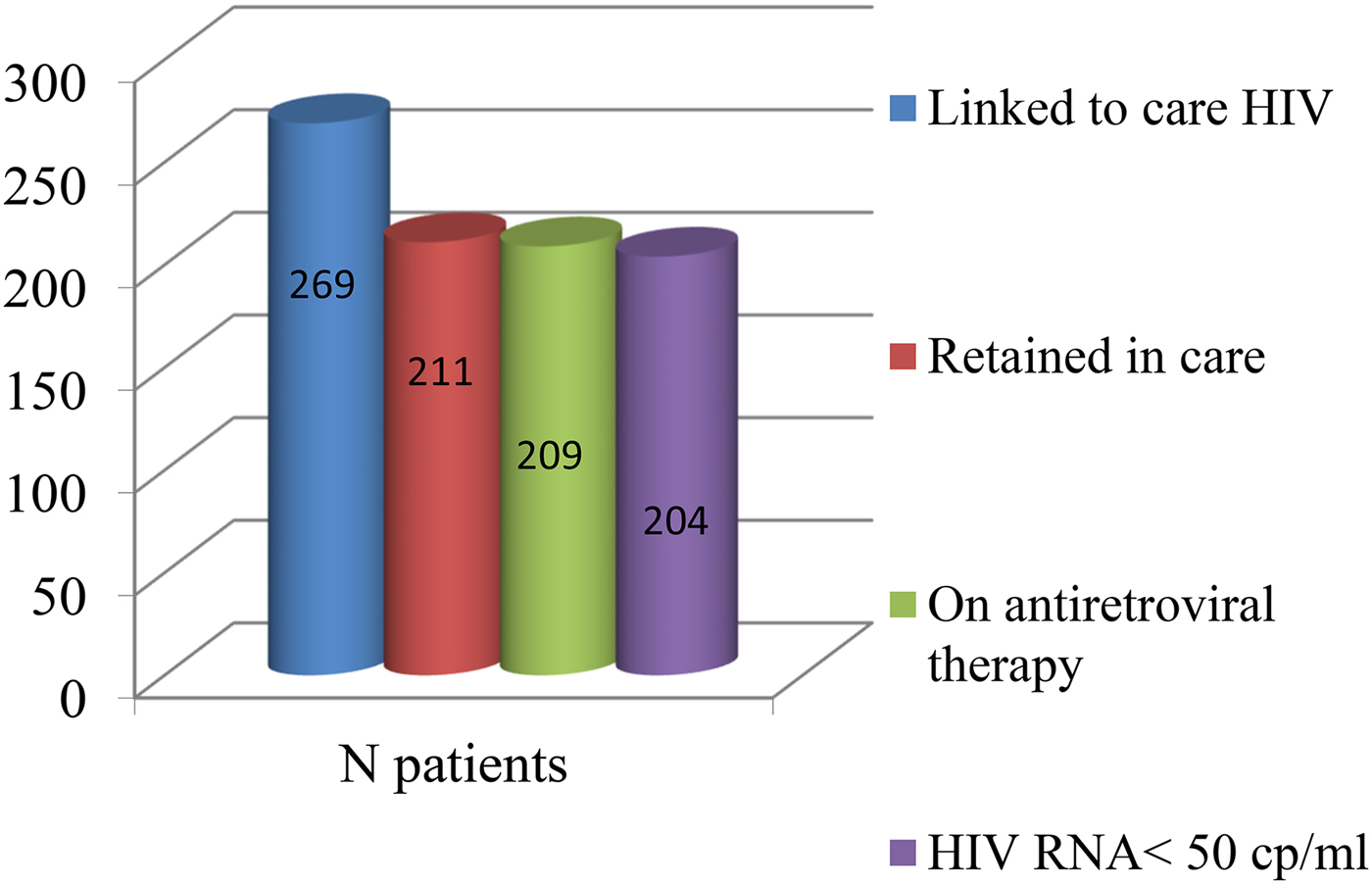
Fig. 1. The cascade of continuum of care.
Table 1. Demographics data and difference between retained and not retained in care patients

cART, combined antiretroviral treatment.
In the multivariate analysis, an association between failed retention in care and being migrants (P = 0.048), lack of cART prescription (P = 0.0004) and lower lymphocyte T CD4+ cell count at last observation (P = 0.001) was confirmed as illustrated in Table 2.
Table 2. Predictors of failed retention in care

In Figures 2–4, Kaplan–Meier estimates of failed maintenance in HIV care for statistically significant variant were reported.
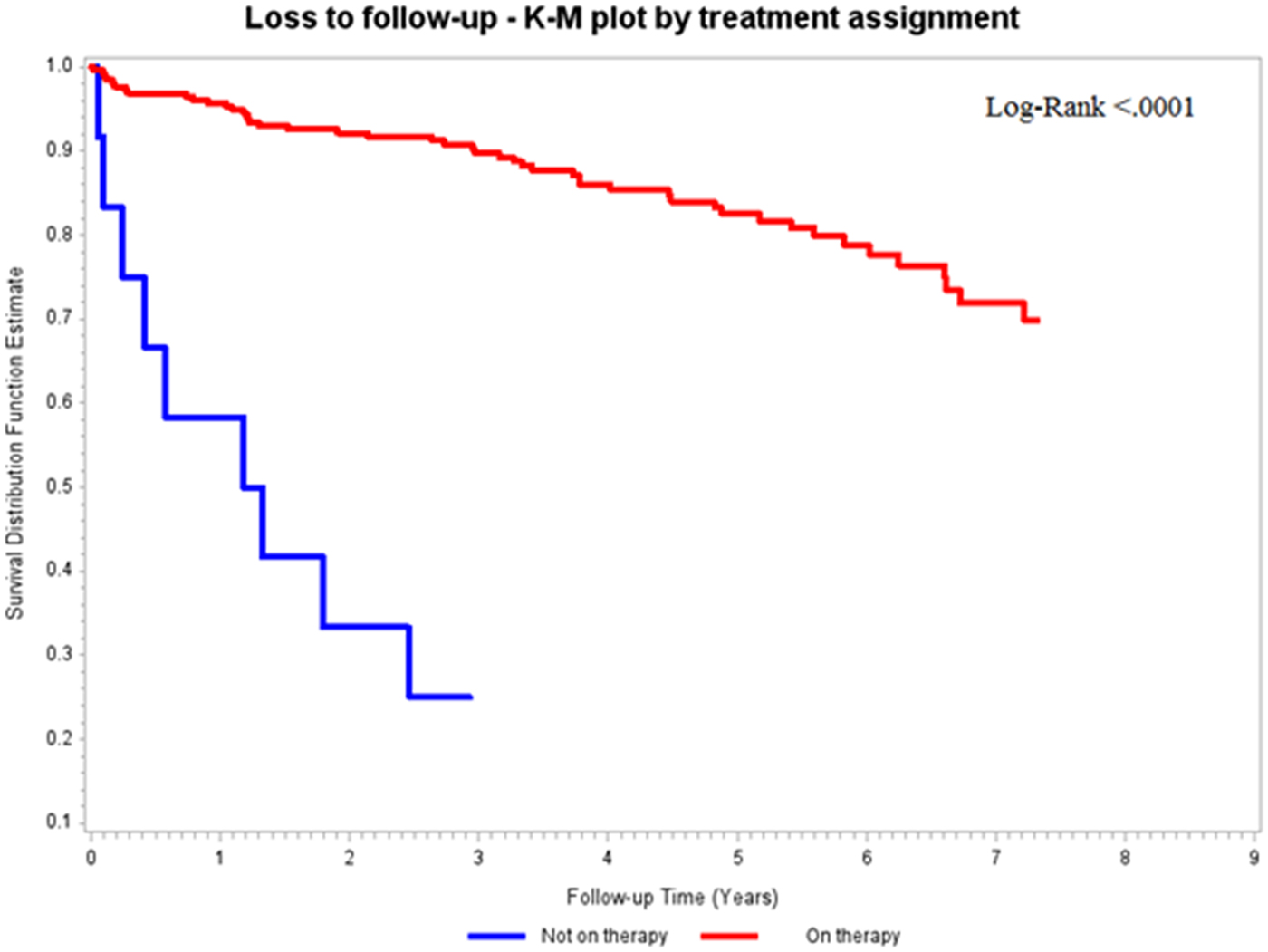
Fig. 2. Kaplan–Meier estimates of failed retention in care according to cART prescription.

Fig. 3. Kaplan–Meier estimates of failed retention in care according to nationality.

Fig. 4. Kaplan–Meier estimates of failed retention in care according to lymphocyte T CD4+ cell count at last observation.
Lastly, predictors of cART efficacy (HIV-RNA <50 copies/ml at last observation) were considered: patients for whom cART was not prescribed were excluded by the analysis (n = 12). In the multivariate analysis, efficacy was lower in the presence of longer HIV disease duration (P < 0.0001) and of baseline HIV-RNA >100 000 copies/ml (P = 0.015) as showed in Table 3.
Table 3. Predictors of failed cART efficacy

Conclusions
The rate of retention in care of HIV-infected patients referring to the Ospedale Policlinico San Martino in Genoa is slightly lower compared with national estimation (78.5% vs. 85%); this is probably attributable to the fact that among the non-caregivers were also included the subjects transferred to other clinical centres within or outside the region.
However, the rate of virological success (rate of patients with HIV-RNA <50 copies/ml on the total of HIV diagnosed patients) was sharply higher (75.8% vs. 58%) [Reference Mammone8]. On the whole, our data, as all Italian ones, resulted superior to estimation in the United States and several other developed countries [7].
In the analysis of predictors associated with failed retention in care, migrant patients were more frequently lost to follow-up compared with Italian subjects. This finding is in agreement with different studies, supporting the concept that HIV-infected migrant subjects are more vulnerable patients due to linguistic, socio-cultural and educational barriers, as well as due to limited knowledge and information about HIV infection and its prevention [Reference Hernando19]; they access later to treatment, with consequent higher rate of late HIV presentation [Reference Saracino20]. Furthermore, foreign HIV-infected patients more frequently exhibit poor adherence to the prescribed care [Reference Lima21, Reference Oh22] requiring proactive therapeutic simplifications [Reference Di Biagio23]. In 2012, published data from Swiss Cohort demonstrated that there was a greater risk of failed maintenance in care among migrant patients with a rate of 8.10/100 person-years for sub-Saharan African males with HIV infection (adjusted risk ratio 2.82, P < 0.0001) [Reference Thierfelder24]. Similarly to migrant patients, subjects diagnosed with more advanced disease, particularly with CD4+ cell count <200/mmc (advanced naives) [Reference Antinori25], were more likely to be lost to follow-up. In our analysis, this result was due to died subjects included in the not retained in care group: notably, the mortality associated with HIV infection is related to the degree of immunosuppression [Reference Mocroft26]: the risk of developing an AIDS-defining condition increases exponentially with the fall of CD4+, particularly below the 200/mmc threshold [Reference Phillips27]. This hypothesis was supported by the trend shown by the Kaplan–Meier curve (Fig. 4) where the scissors expanded immediately, demonstrating that, for the group of patients with low CD4+ cell count, failed retention in care suddenly occurred. Another predictor of high retention in care was the prescription of cART, thus supporting the now-assimilated concepts of ‘test and treat’ and of ‘Therapy as Prevention’. START and Temprano studies [28, 29] indicated that cART was associated with a clinical benefit on progression to AIDS or death even in subjects with CD4+ >500 cells/mmc. Moreover, cART prescription played a role in the reduction of HIV transmission, in the containment of the epidemic [Reference Cohen, Chen and McCauley30] and, on the basis of our findings, was protective against the loss to follow-up. Regarding the analysis of cART efficacy predictors, it emerged that longer duration of disease was associated with failed virological suppression. This was probably justified by the fact that patients who have an older infection, more often have changed the therapeutic regimen over the years, resulting in a greater pill burden and in worse adherence.
A correlation between failed cART efficacy and higher HIV-RNA load at baseline was observed: in the literature is well described that pre-treatment HIV-RNA >100 000 copies/ml is associated with an higher risk of clinical progression and poor response to treatment [Reference Wood31], suggesting that the choice of first-line regimen in the presence of high baseline viral load should be directed towards the use of more potent drugs [Reference Di Biagio32]. In conclusion, our results, in agreement with national ones, are still far from the target of 90-90-90 identified by UNAIDS in 2014, in which it is supposed that diagnosing 90% of HIV-infected patients, treating 90% of them and obtaining 90% of virological suppression by 2020 will result in a reduction of 70–80% of new infections and deaths by 2030 [33, Reference Piot34]. However, identifying categories of patients at greater risk of not being retained in care, such as migrants and advance naïve patients, and intensifying test and treat strategies could facilitate the achievement of the UNAIDS goal [35].









