Introduction
Guillain Barré syndrome (GBS) is an autoimmune disorder of the peripheral nervous system triggered by autoantibodies formed in response to antigenic stimuli [Reference Sejvar1]. Antecedent exposures can include certain vaccinations (e.g. influenza) and viral or bacterial (especially Campylobacter) infections [Reference Hadden2–Reference Sencer and Millar6]. GBS is the most common cause of acute flaccid paralysis worldwide [Reference Sejvar1]; studies in Europe and North America report estimates of GBS incidence of 0.6 to 3.0 cases per 100 000 person-years [Reference Sejvar1, Reference McGrogan7]. GBS is associated with severe morbidity, with patients frequently requiring extended ICU stays and up to 67% experiencing at least one major complication [Reference Henderson8, Reference Dhar, Stitt and Hahn9]. The economic cost is estimated to be $1.7 billion annually in the USA [Reference Frenzen10].
Campylobacter causes an estimated 1.3 million enteric illnesses annually in the USA, making it the most common bacterial cause of gastroenteritis [Reference Scallan11]. Campylobacter jejuni accounts for most Campylobacter infections and has been estimated in various settings and countries to precede 20%–31% of GBS cases with incidence estimated at 20–65 GBS cases per 100 000 Campylobacter infections [Reference Hadden2, Reference Tam12–Reference Mishu and Blaser20]. However, recent estimates for US populations are not available [Reference McGrogan7, Reference Mishu and Blaser20].
Determining the risk of post-Campylobacter GBS is challenging for several reasons. Campylobacter infection is often undetectable by the time GBS symptoms begin because Campylobacter is typically shed for less than 2 weeks after onset of diarrhoea, whereas GBS symptoms typically present between 1 and 3 weeks after diarrhoea onset [Reference Hadden2, Reference Nachamkin and Blaser16, Reference Mishu and Blaser20, Reference Svedhem and Kaijser21]. In addition, due to mild symptoms or asymptomatic infection, many Campylobacter infections go undiagnosed, with an estimated 30 undiagnosed infections occurring for each laboratory-confirmed infection [Reference Scallan11]. Diarrhoea can be mild [Reference Blaser22], so infected persons may not seek care. Even if a stool sample is submitted, Campylobacter can be difficult to detect [Reference Voetsch23]. In the USA, surveillance for Campylobacter infection is conducted by the Centers for Disease Control and Prevention's (CDC) Foodborne Diseases Active Surveillance Network (FoodNet), the foodborne disease component of the Emerging Infections Program (EIP) [Reference Henao24]; however, no routine surveillance for GBS exists [Reference McGrogan7].
An increased risk of GBS following vaccination with a specific formulation of the vaccine targeted at an H1N1 influenza virus was identified in 1976 [Reference Schonberger25, Reference Langmuir26], though no significant increased risk was observed with subsequent seasonal influenza vaccines formulations [Reference Lasky27–Reference Haber29]. However, when a novel influenza A (H1N1) virus similar to the type identified in 1976 emerged in 2009 [Reference Louie30–32], concerns about post-vaccination GBS arose and CDC initiated a special EIP surveillance activity. This surveillance activity, conducted during the 2009–2010 novel influenza A vaccination campaign to assess the risk of post-vaccination GBS found no additional excess risk beyond typical that of seasonal influenza vaccines [Reference Wise33] and offered the opportunity for secondary analysis focused on post-Campylobacter GBS. This included extensive data collection on persons who were determined to not have GBS, providing a unique, well-characterized comparison group. Here, we report an analysis of the association of GBS with laboratory-confirmed Campylobacter infection (the most specific measure for campylobacteriosis) and with diarrhoeal illness (the most sensitive, available measure) to estimate the fraction of GBS attributable to laboratory-confirmed Campylobacter infection or diarrhoeal illness. We also present estimated rates of post-Campylobacter GBS.
Methods
EIP GBS special surveillance activity
We used data from the EIP GBS surveillance activity conducted during the 2009–2010 novel influenza A vaccination campaign to analyse the association of GBS with diarrhoeal illness or laboratory-confirmed Campylobacter infection and to calculate the fraction of GBS attributable to diarrhoeal illness or laboratory-confirmed Campylobacter infection.
EIP GBS special surveillance activity population
The EIP includes ten sites and a population that is approximately representative of the US population with respect to demographic and other health indicators, such as poverty (http://www.cdc.gov/ncezid/dpei/eip/). The catchment area for the GBS surveillance activity included 44.9 million persons. Data were collected between 1 October 2009 and 31 May 2010, yielding 22.9 million person-years under surveillance [Reference Wise33]. Possible GBS cases were identified by exhaustive, active, population-based case-finding to identify every resident of the catchment area presenting with symptoms possibly consistent with GBS. This case-finding was conducted through several avenues, including a network of clinicians (e.g. neurologists, clinical pharmacists, other providers), review of hospital admission and discharge data for the International Classification of Diseases-9-Clinical Modification code for GBS (357.0; acute infective polyneuritis) and monitoring of the Vaccine Adverse Events Reporting System. For additional details, see Wise et al. [Reference Wise33].
GBS and non-GBS diagnoses
Data were collected by review of inpatient and outpatient medical records for all possible GBS cases identified with onset of symptoms during the surveillance period [Reference Wise33]. After data collection and review, all possible GBS cases were classified using the Brighton Collaboration criteria for GBS, a classification of diagnostic certainty [Reference Sejvar34]. Cases were classified as confirmed (meeting Brighton level 1 or 2 criteria) or probable (Brighton level 3 criteria) based on clinical, cerebrospinal fluid and electrophysiologic criteria. We considered cases that did not meet the Brighton criteria for levels 1, 2, or 3 or cases in which an alternative diagnosis was reported as non-GBS controls.
Antecedent illness
Information about signs, symptoms and infections experienced in the 42 days before presentation, including diarrhoea, influenza-like illness (ILI), upper respiratory tract infection (URI) and laboratory-confirmed Campylobacter infection, was collected for all of the reported possible GBS cases, including persons ultimately determined to not have GBS. GBS is known to be strongly associated with Campylobacter infection but less with other common causes of diarrhoeal illness, so we examined the association of antecedent illness with GBS diagnosis using five definitions that ranged from highly specific and less sensitive to highly sensitive and less specific for Campylobacter infection. The most specific, least sensitive definition was laboratory-confirmed Campylobacter infection. The most sensitive, least specific was any diarrhoeal illness, which, as described above, was used because Campylobacter infection is usually not laboratory-confirmed. Three additional definitions of intermediate specificity and sensitivity included diarrhoea without ILI, diarrhoea without URI and diarrhoea without either ILI or URI. These were used because ILI and URI can also precede GBS and can sometimes include diarrhoea [Reference Tam12].
FoodNet
FoodNet, the foodborne diseases component of the EIP, is a collaboration among CDC, ten state health departments, the US Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS) and the Food and Drug Administration (FDA). It conducts active, laboratory-based surveillance for selected pathogens transmitted commonly by food, including Campylobacter and publishes annual estimates of incidence. The FoodNet population is similar though not completely identical to the 2009–2010 GBS surveillance population. Based on 2010 US Census data, about 18% of the FoodNet surveillance population resided in areas not included in the EIP catchment and about 15% of the EIP GBS special surveillance population was not included in the FoodNet catchment. We used FoodNet data on laboratory-confirmed Campylobacter infections reported from 15 September 2009 to 14 September 2010. Since the EIP GBS surveillance activity did not cover a full year, we used FoodNet data from 2009 to 2010 on the timing of laboratory-confirmed Campylobacter infection in our extrapolation from 8 to 12-month estimates. Thus, we calculated the proportion of laboratory-confirmed Campylobacter infections reported to FoodNet that occurred during 15 September 2009–15 May 2010, a period shifted 2 weeks earlier than the GBS surveillance activity, to account for an average 2-week lag between onset of Campylobacter-related diarrhoea and onset of GBS.
Statistical analyses
Confirmed and probable GBS cases (Brighton 1–3) were compared with non-cases to determine whether antecedent illness, as determined using the five definitions detailed above, was more common in cases. We calculated odds ratios (OR) to evaluate the association between each definition of antecedent illness and GBS and we used these OR to estimate the attributable risk (AR) [Reference Jewell35].
The AR estimates, in turn, were used to estimate the number of post-Campylobacter and post-diarrhoeal GBS cases that occurred in the EIP GBS surveillance activity population during the surveillance period. Next, incorporating the national estimate of Campylobacter incidence data, we estimated national rates of post-Campylobacter (post-diarrhoeal) GBS in the USA using each of the five definitions of antecedent illness. All analyses were performed in SAS 9.3 (Cary, NC), Microsoft Excel, or the R Package, epiR.
For sensitivity analysis, we also used a more specific definition of GBS limited to confirmed GBS (Brighton levels 1 and 2). We also repeated analyses excluding the 11% of patients referred for possible GBS who had a previous history of GBS.
Results
EIP GBS special surveillance activity
GBS and non-GBS diagnoses
The GBS surveillance activity identified 638 persons with possible GBS, of whom 398 were determined to have confirmed (Brighton levels 1 or 2, n = 349) or probable (Brighton level 3, n = 62) GBS. The other 227 patients were classified as not cases of GBS and served as controls. These included persons whose illness did not meet the criteria for Brighton levels 1–3 and persons who received another diagnosis. These other diagnoses were not collected systematically but included cancer or cancer-related treatment (N = 8), cardiac-related conditions (7), conversion disorder/seizures (6), radiculopathy (6), drug or alcohol abuse (4), chronic obstructive pulmonary disease (3), diabetes-related conditions (3), stroke (3), multiple sclerosis (2), renal failure (2) and other conditions.
Antecedent illness
Complete antecedent illness reports were available for all 638 patients (Fig. 1, Table 1). From most sensitive to most specific for Campylobacter infection, antecedent illnesses in the 42 days before onset of symptoms of possible GBS included, 79 (12%) with diarrhoea, 68 (11%) with diarrhoea without ILI, 63 (10%) with diarrhoea without URI, 55 (9%) with diarrhoea without ILI or URI and 6 (1%) with laboratory-confirmed Campylobacter infection. The number of laboratory-confirmed Campylobacter infections was, as expected, substantially smaller than for the other antecedent illness definitions, though generally consistent with the other definitions (Table 1). Therefore, we focus on the other, more sensitive, antecedent illness definitions. Estimates of association with GBS ranged from OR = 3.2–4.2. Attributable risk percent ranged from 8.2% to 12.3%, indicating that 33.7 to 50.5 of the 411 GBS cases diagnosed in the EIP GBS surveillance were attributable to Campylobacter infection, as measured by the various antecedent illness definitions (Table 1).

Fig. 1. Diarrhoea, influenza-like illness (ILI) and upper respiratory illness (URI) during the 42 days before onset of symptoms of possible Guillain Barré syndrome (GBS), Emerging Infections Program GBS surveillance, October 2009–May 2010.
Table 1. Association of antecedent illness, based on definitions ranging from highly sensitive to highly specific for Campylobacter infection and association with Guillain Barré Syndrome (GBS) (Brighton Criteria 1–3)
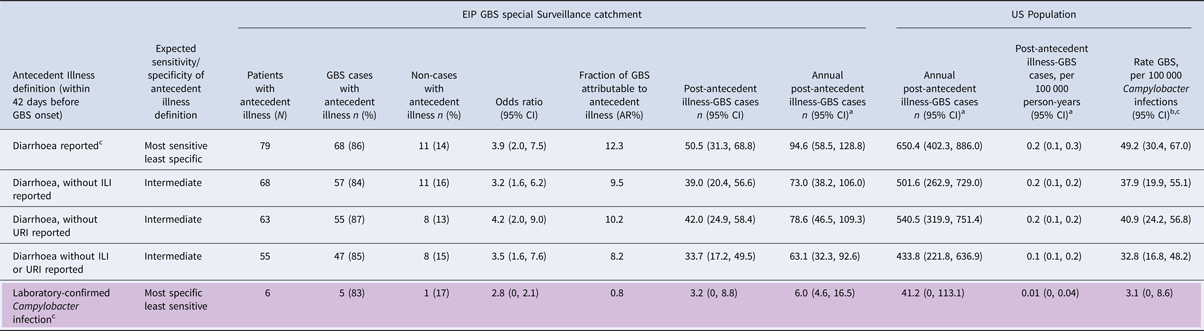
a Results of main analyses extrapolated using 2009–2010 FoodNet data.
bResults of main analyses extrapolated using annual estimates for Campylobacter infections [Reference Scallan11].
c Estimated 1 in 30 Campylobacter infections are laboratory confirmed [Reference Scallan11].
FoodNet
From 15 September 2009 through 15 May 2010, 3394 cases of Campylobacter infection were reported in FoodNet, representing 53% of all Campylobacter cases reported to FoodNet during the 1-year period from 15 September 2009 to 14 September 2010 (N = 6353). Applying this proportion to the estimate for each antecedent illness definition shows that, for the more sensitive case definitions (i.e. definitions based on symptomatology rather than laboratory-confirmation) an estimated 63.1 to 94.6 attributable GBS cases occurred in the EIP catchment population during the 1-year period from 1 October 2009 to 30 September 2010 (Table 1). Extrapolating from the EIP population, an estimated 433.8 to 650.4 post-Campylobacter GBS cases occurred in the USA during this 1-year period, yielding a rate of 0.1 to 0.2 cases per 100 000 person-years. Using our 1-year estimates of post-antecedent illness GBS and the 1-year estimate of Campylobacter infections (1 322 137infections) [Reference Scallan11], approximately 32.8 to 49.2 cases of GBS occurred for every 100 000 Campylobacter infections in the USA. Table 1 also shows the lower estimates obtained using the highly specific definition of laboratory-confirmed Campylobacter infection; they are in the expected range, given the known underreporting of Campylobacter infection.
Assessment of more or less specific definitions
Analyses repeated using the more specific GBS case definition (confirmed cases only) and excluding persons with a previous history of GBS yielded similar results (data not shown).
Discussion
High quality, comprehensive, population-based, active surveillance data are rarely available for GBS, which, though uncommon, is responsible for high morbidity and economic burden [Reference Henderson8–Reference Frenzen10]. We conducted a secondary analysis of GBS surveillance data collected during the 2009–2010 novel influenza A vaccination campaign to generate contemporaneous estimates of the burden of GBS attributable to Campylobacter infection in the USA; the primary analysis demonstrated that the risk of GBS following novel H1N1 vaccination was extremely low and not greater than what is typically observed for seasonal influenza vaccines. We estimate that 8.2–12.3% of GBS is attributable to antecedent Campylobacter infection, with 433–650 cases of GBS occurring annually in the USA (32.8–49.2 per 100 000 Campylobacter infections) that are attributable to antecedent Campylobacter infection. Although attributable risk estimates are at the lower end of the range of previous estimates for the USA and other developed countries, the incidence estimates are in the mid- to upper- range [Reference McCarthy and Giesecke17–Reference Mishu and Blaser20].
The EIP GBS surveillance activity provided a unique opportunity to investigate the association between Campylobacter and GBS. Strengths of the analysis include detailed health history collected through intensive, active, population-based surveillance not only from individuals who met the GBS case definitions but also from a comparison group. Given that Campylobacter infection is usually not laboratory-confirmed (only six laboratory-confirmed cases were reported) and diarrhoea often resolves before GBS symptom onset [Reference Hadden2, Reference Nachamkin and Blaser16, Reference Mishu and Blaser20, Reference Svedhem and Kaijser21], the collection of signs and symptoms in the 42 days prior allowed exploration of multiple definitions of varying sensitivity and specificity to represent antecedent Campylobacter illness. Of note, although the catchment areas of the EIP GBS surveillance activity and FoodNet did not perfectly overlap and Campylobacter incidence estimates were geographically contingent, the low proportion of mismatch in the catchment areas would not be expected to lead to a large difference in our results.
Campylobacter is the most common bacterial cause of domestically-acquired acute gastroenteritis in the USA [Reference Scallan11, 36]. With rare exceptions [Reference Rhodes and Tattersfield3, Reference Eltayeb and Crowley37], the other top three acute gastroenteritis pathogens (norovirus, Salmonella and Clostridium perfringens) have not been consistently associated with GBS. However, the less specific but more sensitive definitions of antecedent Campylobacter illness based on diarrhoeal symptoms may have misclassified other diarrhoeal infections that are rare antecedents of GBS. The impact of these biases is hard to predict. On one hand, using diarrhoea as a proxy for campylobacteriosis should overestimate antecedent illness in both cases and controls, leading to underestimation of the association between Campylobacter infection and GBS. On the other hand, to the extent that other diarrhoeal syndromes are truly associated with GBS, attributing them to Campylobacter would lead to an overestimate of the post-Campylobacter association.
A limitation is that some patients with Campylobacter infection may not have reported diarrhoea. For example, one patient with culture-confirmed Campylobacter infection did not report diarrhoea and therefore was not captured by the diarrhoeal definition of antecedent illness. However, GBS diagnosis (case vs. non-case) would not have influenced testing for Campylobacter or report of diarrhoea in the previous 42 days because these occurred before the onset of the symptoms that led to reporting of possible GBS. In addition, identification of Campylobacter infection was limited to reported symptoms and clinical culture; serological testing was not performed. This may have led to underreporting of Campylobacter infection, thus underestimating the reported association.
Campylobacteriosis was not nationally notifiable at the time of the EIP GBS surveillance project. Therefore, a major strength of using FoodNet special surveillance data for the annual incidence of Campylobacter infection in the USA is that the data were collected through active laboratory-based surveillance, which estimates infections and incidence rates more accurately than passive surveillance. The estimated annual incidence of campylobacteriosis was generated using 2006 data, while the EIP GBS special surveillance covered an 8-month period during 2009–2010. This is unlikely to have substantially affected our results, as the incidence of Campylobacter infection remained relatively stable between 2006 and 2010 [Reference Crim38].
This analysis provides updated estimates related to GBS cases following Campylobacter infection in the USA. Post-Campylobacter GBS tends to be more severe than GBS following other antecedent events, with worse outcomes and slower recovery [Reference Rees14]. Campylobacter infections in the USA have an estimated economic burden of ($1.9 billion), over half which is attributed to GBS-related morbidity and mortality [Reference Hoffmann, Maculloch and Batz39]. Efforts to decrease Campylobacter infections, a priority of the Food Safety Modernization Act, would likely contribute to a decrease in GBS, specifically the most severe GBS cases, thereby substantially mitigating morbidity and mortality associated with Campylobacter infection.
Acknowledgements
We gratefully acknowledge Patricia M. Griffin for the initial idea for the analysis and for helpful suggestions on the manuscript. We also thank the many Emerging Infections Program staff for their contributions to the active, population-based surveillance during the 2009–2010 novel Influenza A (H1N1) vaccination campaign. This work was not supported by grant funding from any agency, commercial or non-for-profit organization.
Conflict of interest
None.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.





