The emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and its related disease (COVID-19) [Reference Huang1] has significantly affected the haemodialysis population [Reference Rombola2–Reference Li and Xu4]. Transmission is thought to be mainly by respiratory aerosols, although it is becoming evident that asymptomatic carriers also play an important role [Reference Bai5] and the presenting symptoms can be non-respiratory [Reference Ferrey6]. We report our experience with the management and follow-up of contacts in a large university-associated, in-hospital dialysis centre.
Our haemodialysis centre is one of the biggest in the country and provides a service to approximately 200 out-patients, as well as to all in-hospital patients, in three regular shifts and in an on-demand midnight shift. There are five dialysis rooms, each with five–seven dialysis beds, and two separate isolation rooms (with two and six beds) with a separate entrance. The isolation rooms are used for the dialysis of hepatitis or HIV positive patients, as well as for those colonised with multi-drug resistant bacteria. Staffing comprised of two nurses per room (i.e. approximately one nurse per three beds). Dialysis for COVID-19 positive patients was provided with nurses and monitors for acute dialysis on a one-to-one basis in the isolation ward of the Department of Infectious Diseases. The isolation rooms had double doors, but were not under negative pressure. All SARS-CoV-2 positive cases were confirmed using reverse transcription polymerase chain reaction (PCR) testing of a nasopharyngeal swab.
On 21 March 2020, a hospitalised haemodialysis patient (patient #1) tested positive for SARS-CoV-2 virus after another patient from the same room on the hospital ward had tested positive. Patient #1 was without fever or respiratory symptoms, and was referred to the COVID-19 Department and dialysed in isolation. All patients dialysed at least once in the same room at the Dialysis Centre in the prior week were considered contacts (Fig. 1), and were quarantined in their dialysis room (i.e. no other patients were dialysed in that room in the same shift). On 14 March 2020, 1 week prior to the emergence of the first patient, a decision to start the universal use of surgical masks (type IIR) by dialysis personnel was made at the hospital level. Patients with symptoms and those who had travelled abroad or been in contact with COVID-19 patients were instructed to call ahead to arrange for testing. At the Centre's entrance, screening personnel asked patients these same questions and measured their temperature. On 22 March 2020, after the first positive patient emerged, all patients began to wear surgical masks throughout dialysis, and contacts also wore masks during transport to dialysis. The nurses dialysing patients in quarantine also used eye protection and wore gowns, caps and gloves. On 26 March 2020, patient #2 tested positive after being discharged as asymptomatic from the Surgery Department on 18 March 2020, with an outbreak of COVID-19 developing on 20 March 2020, after her discharge. The patient had a persistent mild cough, which went unnoticed by the personnel and herself, and then tested positive after developing a fever. In addition to in-centre contacts (Fig. 1), three patients were transported to dialysis in the same vehicle without wearing masks. On 29 March 2020, patient #3 tested positive. He had been dialysed in the same room together with patient #1 16–11 days beforehand, and had also been driven to dialysis together with patient #2 up to 4 days before testing.
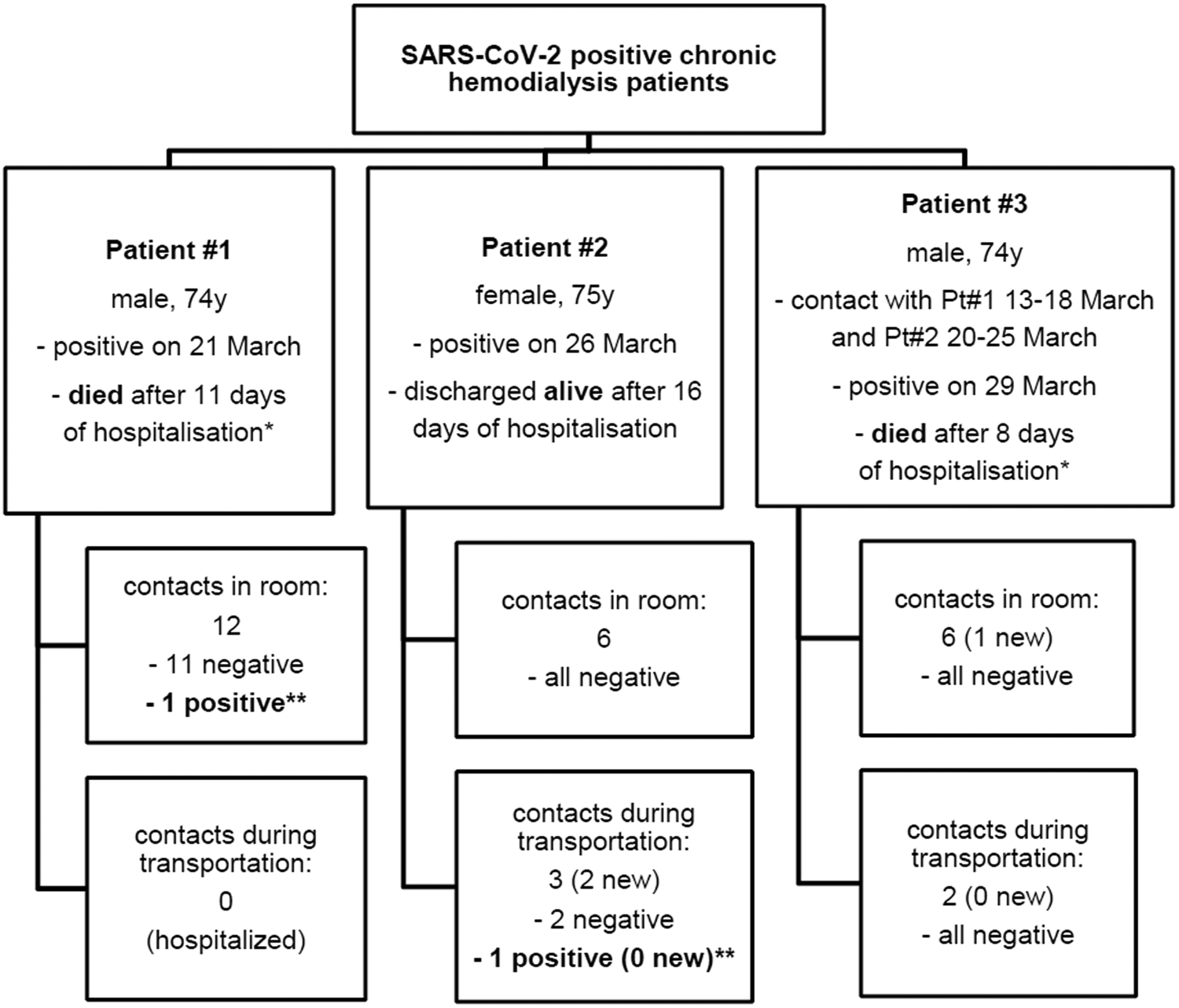
Fig. 1. Summary of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission from three positive patients to their contacts either in haemodialysis (HD) room or during transportation to dialysis centre. Out of 21 different patient-contacts only one tested positive for SARS-CoV-2 within 14 days after last contact. For personnel-contacts, see main text. *Severe comorbid conditions. **The positive patient is case #3.
Altogether 21 patients, 22 dialysis nurses and seven doctors were considered as contacts (Fig. 1). The closest contacts (three nurses and two doctors) were put in home self-isolation for 14 days, while others continued to work using personal protective equipment and following strict self-observation instructions. Given the epidemiological situation, the personal protective equipment used by personnel was upgraded: FFP2 masks, protective glasses, gowns, caps and nitrile gloves were used along with strict disinfection of surfaces and hand hygiene. No new cases emerged among patients or personnel during the next month on the background of a well-controlled epidemic at a national level. All patient and personnel contacts underwent PCR testing of nasopharyngeal swabs approximately 1 week and at least 2 weeks after the last contact; all, except patient #3, remained asymptomatic and tested negative. In addition to the organised management of contacts, 15 patients without in-centre contact were sent for nasopharyngeal swab testing in this 5-week period due to symptoms suggestive of COVID-19; all of them tested negative. Two of three infected patients, of advanced age and with comorbid conditions, died.
Many contacts occur between dialysis patients (during transport, within the dialysis unit and for those in in-hospital facilities also with patients hospitalised in other departments) and with personnel, which represents a relatively high risk for the rapid transmission of SARS-CoV-2. In case of a disease outbreak among personnel, the ability of the centre to offer dialysis can be severely compromised. Therefore, an active approach – identifying symptomatic patients prior to their arrival for scheduled dialysis – is needed [Reference Rombola2–Reference Li and Xu4]. When readily available, a low threshold for testing should be used [Reference Rombola2, Reference Basile3]. Reports from Lombardy, Italy [Reference Rombola2] and Wuhan, China [Reference Li and Xu4, Reference Su7] indicate that a large proportion (10–30%) of dialysis centre patients can become infected, although it is unclear whether this was due to in-centre dissemination or the result of an overall high prevalence in the population. Nevertheless, after the isolation of positive patients, no new cases were reported in Lombardy after a week or so [Reference Rombola2]. More in line with our observations, another centre in Lombardy [Reference Rombola2] and one in Wuhan [Reference Wang8] reported a much smaller number of infected patients (approximately 2–3%) and no in-centre spread, while data from a paediatric centre suggest some transmission occurred between patients within the unit [Reference Hains9]. Therefore, transmission occurring as a result of contact during in-centre dialysis, at least with asymptomatic or oligosymptomatic patients, seems unlikely given an appropriate distance between dialysis stations, in particular if surgical masks are used by both patients and personnel.
Furthermore, group transportation to dialysis, when provided for and covered by health insurance, as is the case in our country, especially in the absence of appropriate protective measures, seems to be the Achilles heel. Given the proximity and timing of contact, we believe patient #3 became infected during transportation to dialysis together with patient #2, and not during dialysis in the same room with patient #1. Whenever possible, transport should be individualised or provided by the patient's next of kin. Notably, and in accordance with some other reports [Reference Rombola2, Reference Li and Xu4], transmission to personnel is very unlikely, if appropriate measures, including surgical masks, are used. Although a 44% subclinical seroconversion rate for personnel has been reported in a paediatric centre [Reference Hains9] using the same level of protection, this is possibly due to more close contacts with children. Community transmission could not be excluded, since the seroconversion rate for personnel in direct contact with two subclinical seroconversion patients was modest (2/11, 18%) and only one had a positive nasopharyngeal swab [Reference Hains9].
To conclude, we believe that the universal use of at least surgical masks by personnel and patients during dialysis and group transportation offers the most optimal protection. Active identification of symptomatic patients, nasopharyngeal swab testing, strict isolation of positive cases, as well as quarantine and follow-up of contacts, are powerful tools to restrict transmission in dialysis units [Reference Basile3, Reference Li and Xu4, Reference Hellewell10]. Fortunately, with the use of these precautions, SARS-CoV-2 transmission in relation to the provision of haemodialysis treatment seems to be very low.
Financial support
This work was supported by the Slovenian Research Agency (research core funding No. P3-0323).
Conflict of interest
None.
Data availability statement
All the data are included in the paper.




