INTRODUCTION
Meningococcal disease (MD) is an important public health problem by virtue of its gravity and epidemic potential [1].
In family and school settings, the proportion of asymptomatic carriers increases with the occurrence of a case [Reference Ahlawat2, 3]; there is no clear relationship between the proportion of carriers in a population and the incidence of disease [4].
Transmission of the meningococcus is favoured by crowding in dormitories, barracks, and lower socioeconomic households, in particular those with poor ventilation and sanitation [4–Reference Stephens, Greenwood and Brandtzaeg6]. Other factors favouring illness include more virulent strains, viral co-infections, poor nutrition, air pollution, and hot and dry climatic conditions [1, 4, Reference Weiss7]. Children aged <5 years are most at risk, but other age groups can be affected, especially in outbreaks [4, 8, 9].
Chemoprophylaxis of contacts prevents secondary cases, although data on efficacy is incomplete [1, 8, Reference Purcell10]. In outbreak settings involving vaccine-preventable serogroups, particularly in institutionalized populations, vaccination is recommended, especially when the primary attack rate exceeds 10 cases/100 000 population over a 3-month period [1, 3, 8]. Effectiveness of control depends on identifying a well-defined, spatially limited population [Reference Granoff, Harrison, Borrow, Plotkin, Orenstein and Offit11, Reference Jackson12].
In Brazil about 3500 cases are reported annually, with a median incidence of 2 cases/100 000 population and a case-fatality rate of 20% (Brazil Ministry of Health, unpublished data). The main serogroups circulating are B and C, with a progressive increase of serogroup C since the 1990s [1].
Two vaccines are available in Brazil. Meningococcal conjugated vaccine for serogroup C, was available for persons with immune deficiencies until 2010. Meningococcal polyssaccharide vaccine (A+C) is indicated to control outbreaks and is approved for use in individuals aged ⩾2 years [1].
Between June and July 2008, 13 cases of MD were suspected in the municipality of Rio Verde, Goias State, in midwestern Brazil, the site of one of the largest food-processing plants in Brazil. At the time of reporting, two deaths had already occurred. The objectives of our investigation were to identify risk factors for illness and recommend prevention and control measures.
METHODS
Population and study location
Rio Verde has a population of 139 200 inhabitants [Brazilian Institute of Geography and Statistics (IBGE), 2007] and has two well-defined seasons: dry (May–October) and rainy (November–April). Median annual temperature varies between 20°C and 35°C.
The city has 11 community healthcare centres and eight primary-care teams, covering 20% of the population, and three hospitals, one public. There are eight adult, and no paediatric intensive-care beds; severely ill children must be sent to the state capital, 220 km distant.
Studies and definitions
Retrospective case-finding
We conducted a retrospective search for all cases reported as suspected bacterial meningitis between 1 January and 31 August 2008. Suspected cases were those with one or more symptoms and signs compatible with bacterial meningitis. Reporting criteria for suspect cases were as follows: children aged >1 year and adults with fever, intense headache, projectile vomiting, nuchal rigidity, indicators of meningeal irritation (Kernig and Brudzinski signs), convulsions or erythematous rash; for children aged <1 year, irritability such as persistent crying, and bulging fontanelles [1].
Descriptive study
We conducted interviews with healthcare providers, family members and close contacts, and administered a standard questionnaire to the patient whenever possible. A confirmed case of MD was defined as isolation of N. meningitidis by culture of a sterile site, or detection of polysaccharide antigen in a sterile site by latex agglutination, counter-immunoelectrophoresis or polymerase chain reaction (PCR). A probable case was defined as a reported case with an epidemiological link to a laboratory-confirmed case but without positive laboratory results, and a possible case was a patient who presented clinical purpura fulminans without specific laboratory results, in Rio Verde municipality between June and August 2008.
Case-control study
Only primary household (index case from each household) and confirmed cases of MD were included in a study of risk factors for disease. Each case was matched to four controls by residence; controls were neighbours who did not have a history of MD in the family during the study period. Control households were identified in homes located sequentially to the right of the case's home. Within households, controls were randomly selected from a list of household members using a random numbers table.
Laboratory testing
Cerebrospinal fluid (CSF) and blood samples were collected for culture, latex agglutination and counter-immunoelectrophoresis at local and state laboratories, according to a standardized procedure [Reference Popovic, Ajello and Facklam13].
Serogrouping
The serogroup was confirmed in all N. meningitidis isolates by slide agglutination [Reference Popovic, Ajello and Facklam13] with polyclonal goat or horse antisera prepared at Adolfo Lutz Institute (Sao Paulo, Brazil) against serogroups A, B, C, W135, X, Y and Z, as described previously [Reference Alkmin14, Reference Mothershead15].
DNA extraction
DNA from 13 CSF samples was extracted and purified using QIAamp DNA Mini kit (Qiagen, USA) according to the manufacturer's instructions.
Reverse transcriptase (RT)–PCR
DNA amplification using TaqMan® probe systems was performed by RT–PCR, for detection of N. meningitidis capsular transport (ctrA) genes [Reference Mothershead15], pneumococcal autolysin gene (lytA) for Streptococcus pneumoniae [Reference Carvalho16], polysaccharide capsular expression gene (bexA) to Haemophilus influenzae [Reference Corless17] and genogrouping B, C and W135 of Neisseria meningitidis using capsular biosynthesis gene as target siaD (B and C) and synG (W135) [Reference Mothershead15]. The RT–PCR reactions were performed and analysed using the 7300 ABI PCR system (Applied Biosystems, USA). Samples were deemed positive when showing an amplification curve and multicomponent characteristics with a cycle threshold (Ct) cut-off value of ⩽38.
Serological typing
Serotyping for five isolates, three from the period of the investigation and two post-investigation, was performed by dot-blotting using whole-cell suspensions as described previously [Reference Wedege18]. The serotyping was performed with a set of 18 PorB and 15 PorA murine monoclonal antibodies (MAbs) specific for the variable regions [Reference de Lemos19].
Phenotypic antimicrobial susceptibility testing
The susceptibility of meningococcal strains to penicillin, ampicillin, chloramphenicol, ciprofloxacin, ceftriaxone and rifampicin was analysed by determining the minimal inhibitory concentration (MIC) using the broth microdilution procedure [20, 21]. Susceptibility/resistance breakpoints were those recommended by the European Monitoring Group on Meningococci (EMGM) [Reference Vazquez22].
Pulsed-field gel electrophoresis (PFGE)
DNA was extracted from a bacterial suspension adjusted to an OD610 of 1·3–1·4 following incubation with proteinase K. DNA was digested with NheI and separated by electrophoresis in agarose 1% w/v gels as described previously [Reference Popovic23]. Restriction profiles were analysed visually and with Bionumerics software (Applied Maths, Belgium). A dendogram was constructed by the unweighted pair-group method with arithmetic averages. The Dice band-based similarity coefficient (S D) was used with a band position tolerance of 1·5% and an optimization of 1·0%. Isolates were typed by coefficient of similarity. Isolates sharing >85% similarity were considered to be related and were tabulated by using the same capital letter and numbers if the isolates were non-identical but closely related [Reference Tenover24, Reference Hartstein25].
Multilocus sequence typing (MLST)
MLST was performed according to the methods of Maiden et al. [Reference Maiden26]. Primers, determination of sequence alleles, and designation of sequence types are described on the MLST website (http://neisseria.org/nm/typing/mlst).
Statistical analysis
The data were analysed in Epi-Info 3.5.1 (Centers for Disease Control and Prevention, USA) and Microsoft Excel 2003. The measure of association was the Mantel–Haenszel matched odds ratio (mOR) with 95% confidence interval (CI) and α=5%. We tested significance with the McNemar χ2 test.
RESULTS
Retrospective case-finding
During the investigation period, 56 cases of meningitis were reported to public health authorities. Of 22 meeting the suspected case definition for MD, ten were confirmed for serogroup C infection, five met the probable case definition and one met the possible case definition. The other six were discarded as MD. Thus 16 cases were included in the descriptive study, of which 10 were classified as primary cases, five as co-primary cases and one as a secondary case [Reference Bilukha and Rosenstein5].
Outbreak description
Of 16 MD cases, ten (63%) were male; median age was 6 years (range 2 months to 45 years). The first case was reported in early June (epidemiological week 23) and the majority of cases occurred in August (Fig. 1). Clinical presentation included meningococcal meningitis in nine (56%), meningococcaemia in six (38%), and a combination of both forms in one case.
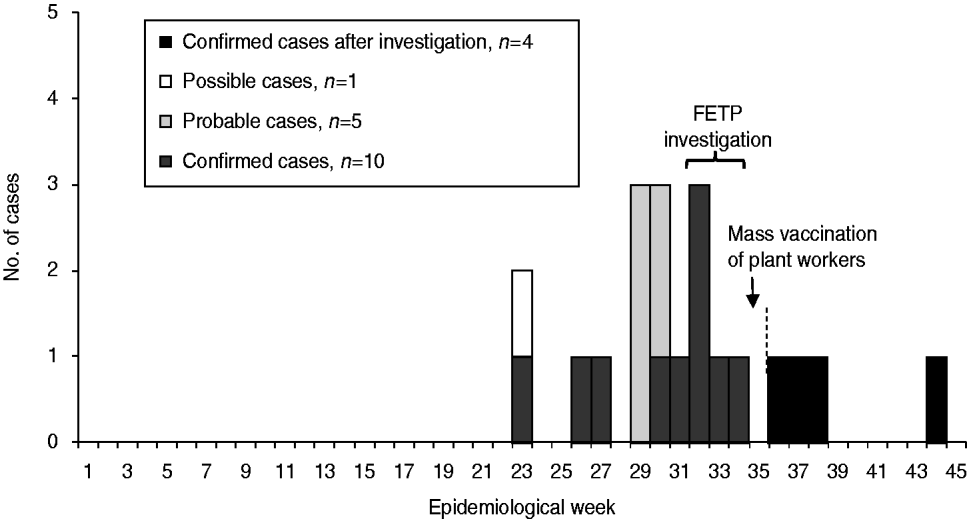
Fig. 1. Case illness onset by epidemiological week, Rio Verde, Goias State, Brazil 2008. FETP, Field Epidemiology Training Programme group.
The attack rate for the municipality was 12/100 000 inhabitants (Table 1). The highest attack rate was in the 1–4 years age group (77/100·000 population), and the case-fatality rate in these cases was 43%.
Table 1. Incidence and case-fatality rate by age of meningococcal disease type C cases between June and August 2008, Rio Verde, Goias State, Brazil
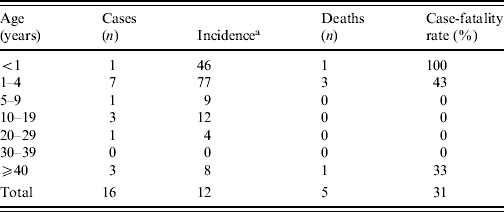
a Number of cases/100 000 inhabitants.
Symptoms and signs presented by confirmed, probable and possible cases included: fever (100%), vomiting (88%), headache (11%), myalgia (7%) and petechiae (7%). Only 5% of cases presented nuchal rigidity. Of eight children aged <8 years, four presented persistent crying and irritability. There were no reports of bulging fontanelles, coma or Kernig or Brudzinski signs. Of eight cases that presented with petechiae, four died.
All 16 case-patients sought medical care; 11 (69%) were hospitalized and eight (50%) were placed in intensive care. A median of two medical encounters took place before the diagnosis of meningitis was made (range 1–3); one case died without receiving a clinical diagnosis of MD. Five (31%) patients died and five (31%) had sequelae: three had hearing impairment, one had visual impairment, and one had impaired gait. The median time from onset to recovery was 10 days (range 1–40 days) and the median time from onset to death was <24 h (range 1 h to 31 days). The median time from medical consultation to reporting was <24 h (range 0–3 days) and to prophylaxis initiation 1 day (range 0–5 days). The secondary case did not receive prophylaxis for contact with the primary case in the household.
Cases were dispersed throughout township neighbourhoods. Six cases occurred in one household of 14 persons, constituting an intra-domiciliary outbreak with an attack rate of 42% (one primary, five co-primary, one secondary case). In a second household of five persons, two brothers aged 3 years and 2 months, respectively, fell ill (attack rate 40%) and both died. In households with cases, the median number of residents was six (range 3–14) and the median household income was US$1130 (range US$283–1695).
Of the cases investigated, 14 had direct or indirect exposure to a large food-processing plant. Five were plant employees, one was the driver of the plant employees' transport bus, and the remaining eight were household members of plant workers who developed no symptoms. Of the five plant workers, three worked on the first shift, one on the second shift and one worked hours overlapping first and second shifts; all were posted at unrelated sectors within the plant. The attack rate in the food-processing plant was 60/100 000 employees. We were unable to show elevated risk for workers at any sector or shift.
The food-processing plant in Rio Verde municipality
The plant employed 10 012 persons or 7% of the municipality's population. Most (58%) workers were aged 20–30 years, coming from different regions of Brazil. Worker turnover is about 300 per month. The plant is characterized by closed work areas consisting of four subterranean levels accessible by tunnels, without natural ventilation. Animal delivery, slaughter and deboning areas are hot and humid. The workers' workload averages 10 h/day, 6 days/week.
The median number of workers in the sectors in which cases occurred was 20 (range 17–100). Masks were required only in the sector where final product is processed. We noted crowding of personnel in the plant cafeteria, recreation room, and bus stops.
Clinical laboratory results
Blood and CSF were collected from 22 cases. Samples of ten patients were confirmed by at least one laboratory test for N. meningitidis serogroup C: three by positive culture and seven by RT–PCR, of which three were also positive by counter-immunoelectrophoresis and two on latex agglutination.
Serotyping, MIC and MLST
The five N. meningitidis serogroup C isolates displayed only one serotype–serosubtype antigen combination, C:23:P1.14–6 and only sequence type (ST) 3780 belonging to ST103 complex (Table 2). All isolates were susceptible to all antibiotics tested.
Table 2. Characteristics of case-patients with laboratory-confirmed invasive meningococcal disease, Rio Verde, Goias State, Brazil 2008
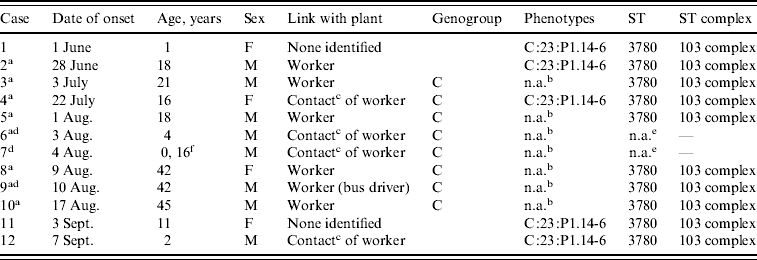
ST, Sequence type; n.a., not available.
a Primary household cases included in analytical study.
b Diagnosis was confirmed by direct-specimen PCR only; no culture isolate was available for serosubtyping.
c Household member.
d Fatal cases.
e Few DNA samples for strain identification.
f 0·16=2 months.
PFGE
Four distinct PFGE profiles were observed, designated by letters and numbers (Fig. 2). PFGE profile A was demonstrated in two isolates with 100% genetic homology, which we designated as the outbreak strain. The isolates were obtained from a processing plant worker and a case-patient who had household contact with four asymptomatic plant workers.

Fig. 2. Dendrogram of meningococcal strains isolated from Rio Verde (Goias State, Brazil) case patients. PFGE, Pulsed-field gel electrophoresis.
PFGE profile A1 was demonstrated in one isolate showing 92% genetic homology with PFGE profile A isolates. It is therefore probably related to the outbreak. This isolate was obtained from the first community case-patient who became ill after the mass vaccination of processing plant workers. We could not establish an epidemiological link to the plant or to other cases.
PFGE profiles A2 and A3 were demonstrated in isolates from two ill children. PFGE A2 was demonstrated in an isolate from a community case-patient during the outbreak; no epidemiological link was established with plant workers. PFGE profile A3 was demonstrated in an isolate from a community case-patient with illness onset 1 week after the mass vaccination at the plant. These isolates had 96% genetic homology to each other and 87% genetic homology to the isolates with the outbreak pattern; they may therefore be related to the outbreak.
Case-control study
Probable and possible cases were excluded and we also had two losses at follow-up. Consequently, the study consisted of eight primary household cases confirmed by at least one positive laboratory test, identified in Table 2, and 32 controls (ratio 1:4). A primary case was defined as the first confirmed case that occurred in the household in the absence of previous known close contact with another MD case. Case households had a median of six residents (range 3–14) while control households had a median of three residents (range 1–6). Six (75%) cases and 11 (34%) controls were male (P=0·04). Exposures significantly associated with illness included: working at the food-processing plant, working in a sector with ⩾20 workers, residing in a self-described poorly ventilated residence, sharing a residence with >4 persons, sharing a bed with one or more persons, and residing in Rio Verde municipality for ⩽1 year (Table 3).
Table 3. Factors associated and not associated with meningococcal disease, univariate analysis, Rio Verde, Goias State, Brazil, 2008 (N=40)
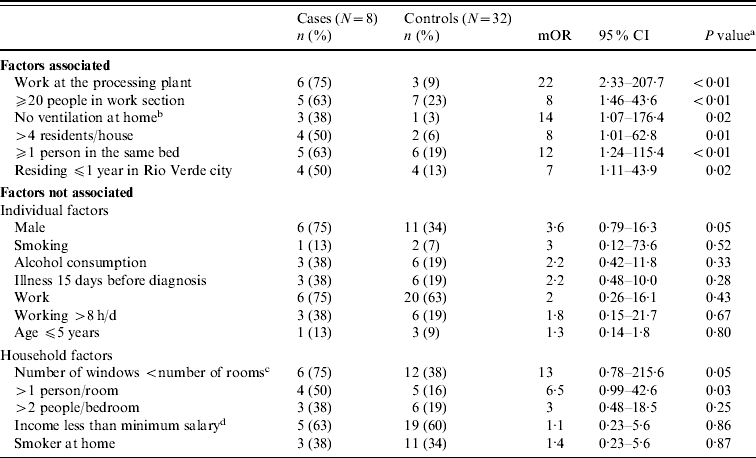
mOR, Matched odds ratio (Mantel–Haenszel); CI, confidence interval.
a Calculated using McNemar χ2.
b Self-reported.
c Window surface area of the home.
d Equal to US$735/month.
Intervention
Household contacts of reported cases received chemoprophylaxis. On 19 August 2008 a decision was taken to vaccinate the 10 012 plant workers; 10 300 polysaccharide meningococcal AC vaccine doses [Immunobiological Technology Institute (Bio-Manguinhos/Fiocruz), Brazil] were provided by the Ministry of Health. Between 22 and 29 August, Rio Verde municipality vaccinated 9200 plant workers. Vaccination took place during work hours on plant property. Eighty mild reactions (local discomfort and erythema) but no severe adverse events were reported.
Following the vaccination intervention, no further cases occurred in the plant workers, but four new cases occurred in the municipality within 2 months. All patients were aged ⩽15 years and three patients had some form of contact with food plant workers (i.e. son, girlfriend, neighbour) (Fig. 1).
DISCUSSION
An outbreak of MD serogroup C occurred in Rio Verde city. Most of the cases occurred in workers (and their contacts) at one of the largest food processors in Brazil. It is likely that crowding and the nature of ventilation at this processor's facilities contributed to disease spread, along with dry and cold seasonal weather, as reported elsewhere [Reference Ahlawat2, 4, Reference Bilukha and Rosenstein5].
This study has several limitations. The most important limitation is the small number of investigated patients in which laboratory confirmation of MD was possible, because of problems in sample collection and laboratory analysis of the earliest cases. We required laboratory confirmation for inclusion in the case-control study, and the resulting small study population was inadequate for age adjustment and conditional logistic regression analysis. Overestimation of the attack rate in infants through inclusion of five probable cases in children from the first household outbreak was a concern. We chose to include only primary cases in the analytical study, recognizing that this decision could bias results in favour of association with the plant, as most confirmed cases were of working age. Additionally, data reported by patients and/or survivors were subject to information and recall biases.
Cases occurred in household contacts of plant workers resulting in two secondary household outbreaks, probably originating with workers who were asymptomatic carriers. This probably explains the high incidence of disease in children, who are more likely to be infected. The residences of these families exhibited poor ventilation and crowding. A massive vaccination effort in the food plant controlled the outbreak in plant workers, with cessation of cases for at least a year. However, new cases occurred in contacts of the workers, illustrating a continuing risk in the community. The similarity in the phenotypes and restriction profile (PFGE) of isolates from outbreak cases and from community cases that occurred after the mass vaccination at the plant confirm this hypothesis. Isolates with PFGE profiles A2 and A3 were possibly genetically related to the outbreak [Reference Tenover24], although we could not identify an epidemiological link between these cases and outbreak cases.
Consistent with earlier studies, males and infants were more affected [4, 8, Reference Baethgen27], but the occurrence of three cases in individuals aged >30 years indicates an age shift, which occurs in MD outbreak situations [Reference Ahlawat2, 8].
The case-fatality rate was similar to that of a recent outbreak in New York City in illicit drug users [Reference Weiss7]. The high morbidity and case-fatality rate can be related to difficulties related to healthcare, including access. The low frequency of classical meningeal signs and symptoms such as nuchal rigidity, especially early in the disease, may have hampered early diagnosis; additionally, waiting time for evaluation and referral of children in need of intensive care to the state capital may have contributed to delays in diagnosis and treatment.
Furthermore, the emergence of a new clone or a clone complex is associated with high attack rates and increased case-fatality rates [Reference Baethgen27]. As previously described, ST103 complex has been present in Brazil since 1989 and became associated with the N. meningitidis C epidemic earlier this decade [Reference de Lemos19]. All strains related to this outbreak have particular PorB and PorA variants that can be characterized as serotype 23 and serosubtype P1.14-6, belonging to the ST103 complex.
The risk associated with duration of residence in Rio Verde may be explained by the same factors that favour illness in the first weeks of military service [Reference Biselli28], such as exposure of susceptible persons to new environments and the introduction of new subtypes [4].
The Brazilian Ministry of Health recommends defining an outbreak of MD by comparison of case counts with previous years [1]. CDC defines an outbreak as the occurrence of three cases caused by the same serogroup within ⩽3 months in persons without direct contact in a defined population [Reference Bilukha and Rosenstein5, 29, Reference Moore and Osterholm30], yielding an attack rate of ⩾10 cases/100 000. Of the plant workers in Rio Verde the incidence was 60/100 000 between June and August 2008, greatly exceeding the proposed CDC threshold [Reference Bilukha and Rosenstein5] and thus justifying a massive vaccination effort. A careful, rapid evaluation of epidemiological data can permit timely application of appropriate control measures in future events [Reference Jackson12, Reference Kaninda31], despite difficulties in establishing criteria and enumerating the population at risk [Reference Weiss7]. Immunization effectiveness in outbreak control varies between 70% and 90% [Reference Ahlawat2, 28, 32–Reference Poland35].
The absence of cases in plant workers in the year following the mass vaccination indicates successful control of the outbreak in the plant. Since MD is endemic in Brazil, and there is no consensus that the polysaccharide vaccine can reduce asymptomatic carriage [Reference Stephens, Greenwood and Brandtzaeg6, Reference Kaninda31, Reference Rosenstein32, Reference Poland35], occurrence of additional cases in the community was not unexpected, including among workers' contacts. A similar situation has been described in recent studies [Reference Weiss7, Reference Biselli28]. Furthermore, the last case among workers had onset before the mass vaccination, and the first case among workers' contacts after mass vaccination had symptom onset 20 days following the last case, more than twice the incubation period of this infection [Reference Ahlawat2]. We could not determine if the workers whose contacts were infected following mass vaccination had worked in the plant at the time of that intervention or if they had been vaccinated.
The effective control by vaccination in the well-defined plant population, contrasted with the persistence of infections in workers' contacts, highlights a challenge to public health officials. The majority of illnesses and deaths from MD in Rio Verde municipality occurred not in workers who were eligible to receive vaccination, but in their family contacts, especially children, who are most vulnerable. On the one hand, these contacts are demonstrably at risk of infection and could also have been a target population for vaccination. On the other hand, the scale of plant workers' contacts extends beyond their households: in the most recent four community cases, three were contacts of plant workers but of these two were not household members. As shown in other MD outbreaks, the definition of the population at risk that should be targeted for vaccination may not be straightforward [Reference Weiss7].
We believe that routine childhood vaccination against serogoup C N. meningitidis could reduce the type of morbidity and mortality seen in this outbreak; this practice has reduced total cases and the severity of cases in Italy, Great Britain, Canada and the USA [Reference Bilukha and Rosenstein5, 8, Reference Biselli28, Reference Begg36]. The World Health Organization recommends consideration of routine use of the conjugated group C vaccine in areas where the disease constitutes a public health problem in young children [8]. Recent assessments of MD in Brazil including such events as this outbreak led to the introduction of routine meningococcal serogroup C conjugated vaccination in Brazil in 2010.
ACKNOWLEDGEMENTS
We thank the local authorities from Rio Verde municipality and Goias State for their support, the health workers for participation in the investigation, and the workers and managers of the processing plant. We also thank the Division of Public Health Laboratories, Central Public Health Laboratory of Goias State and the national and state immunization programmes.
The U.S. Centers for Disease Control and Prevention in cooperation with the United Nations Educational, Scientific and Cultural Organization and Brazilian Ministry of Health provided support for the Field Epidemiology Training Programme.
DECLARATION OF INTEREST
None.







