INTRODUCTION
Measles is an acute viral disease. Although a vaccine has been available since 1959 [Reference Whittle1], measles still remains an important cause of morbidity and mortality in children, particularly in developing countries [2–Reference Bosan4]. In 2000, measles was estimated to have been responsible for the deaths of 777 000 children worldwide, representing about half of the deaths attributed to vaccine-preventable diseases [5]. Measles outbreaks have been reported in refugee and emergency settings [Reference Porter6–Reference Tool and Waldman8] due to mass population displacement, high population density and low vaccination coverage [Reference Morley, Martin and Allen9, Reference Kamugisha, Cairn and Akim10]. For the regional elimination of measles, the World Health Organisation (WHO) and the United Nations International Children's Emergency Fund (UNICEF) advise attaining a high routine vaccination coverage of >90% in every community and ensuring that all children have a second opportunity for measles immunization [11]. Although there are effective measles control strategies [12, Reference Redd, Markowitz, Katz, Plotkin and Orenstein13], case-fatality rates as high as 34% have been reported in refugee settings, while similar rates in stable populations are around 1% [Reference Moore14, Reference Shears15]. In refugees and internationally displaced populations, WHO and UNICEF recommend vaccinating children aged between 6 months and 14 years with a coverage >95%; however, they also advise that the age group to be targeted during such a campaign should be determined based on the local epidemiology of the disease [12]. They also recommend that improvements in case management and surveillance are made to reduce morbidity and mortality associated with this disease.
Rubella is also a viral disease that occurs worldwide with a seasonal pattern and cyclic epidemics every 5–9 years. It is normally a mild disease that occurs in childhood; however, infection during early pregnancy can cause congenital rubella syndrome (CRS) characterized by multiple defects, particularly cardiac, cerebral, ophthalmic and auditory. Differential diagnosis of measles and rubella without laboratory confirmation can be difficult, because both diseases have similar clinical symptoms [Reference Ramamurty16]. Fewer than half of developing countries have introduced rubella immunization into their national immunization programmes [Reference Banatvala17]. Rubella vaccines for childhood immunization are used only in the private sector in many countries, including regions where rubella is not a formal part of the immunization programmes [18]. WHO recommends the elimination of rubella and CRS by universally vaccinating infants and young children using measles-rubella (MR) or measles-mumps-rubella (MMR) vaccines, implementing surveillance, and ensuring immunity in women of childbearing age [18].
Regional conflicts can lead to the displacement of large populations into temporary settlements or camps. The overcrowded and rudimentary shelters, and the inadequate safe water and sanitation increase the risk of infectious disease proliferation [Reference Gayer19]. A number of studies on measles have been conducted; however, few have investigated disaster and displaced populations associated with the disease. The present study describes the epidemiology of concurrent measles and rubella outbreaks in Côte d'Ivoire transit camps (TCs) for Liberian refugees during 2003–2004. It also describes the investigation and experience of a diseases surveillance team (DST) that provided advice to decision makers and public health officials throughout the surveillance and outbreak investigation.
MATERIALS AND METHODS
Background, location and population
Since 1995, West Africa has been the region most affected by conflicts in Africa [Reference Gayer19]. Internal conflicts in Liberia, Sierra Leone, Guinea–Bissau, Guinea, Côte d'Ivoire, Nigeria, Togo and the Casamance Province of Senegal, have resulted in the displacement of millions of people with significant adverse effects on human health [Reference Wyndham20]. Côte d'Ivoire is located in West Africa and is bordered by Liberia to the west. Since 1989, about 70 000 refugees have fled from Liberia to Côte d'Ivoire to escape civil war. They were transferred from the western border areas of Tabou and San Pedro (Fig. 1a) where most of them had been living in large camps or villages, to TCs in Abidjan. The refugees were to be resettled in the USA in 2003–2004 by the International Organization for Migration. A total of 5427 Liberian refugees were distributed into 19 TCs in four municipalities: 13 TCs in Cocody, three in Abobo, two in Koumassi and one in Marcory (Fig. 1b). Compared to normal refugee camps, these TCs had better overall living conditions. Food, clean water and toilet facilities were available, and there was a health unit in each TC supported by two non-governmental organizations (NGOs). Yobou, Biabou, Palmeraie and Eucalyptus TCs were cramped and often overcrowded. Several family groups were living together and sharing rooms and toilets with less than optimal sanitary conditions. Residents of these TCs often visited other TCs. In addition, Biabou and Yobou were continually receiving new refugees. The outbreak of rash and fever started on 19 January 2004. This was 4 days after the first case was detected in the border area prior to the arrival of new refugees at the TCs. Measles and rubella vaccines had not been given to any refugees in the camps prior to outbreaks occurring in Yobou, Biabou, Palmeraie and Eucalyptus TCs (Fig. 1b). Therefore our analysis includes a total of 2767 Liberian refugees living in the four affected TCs.
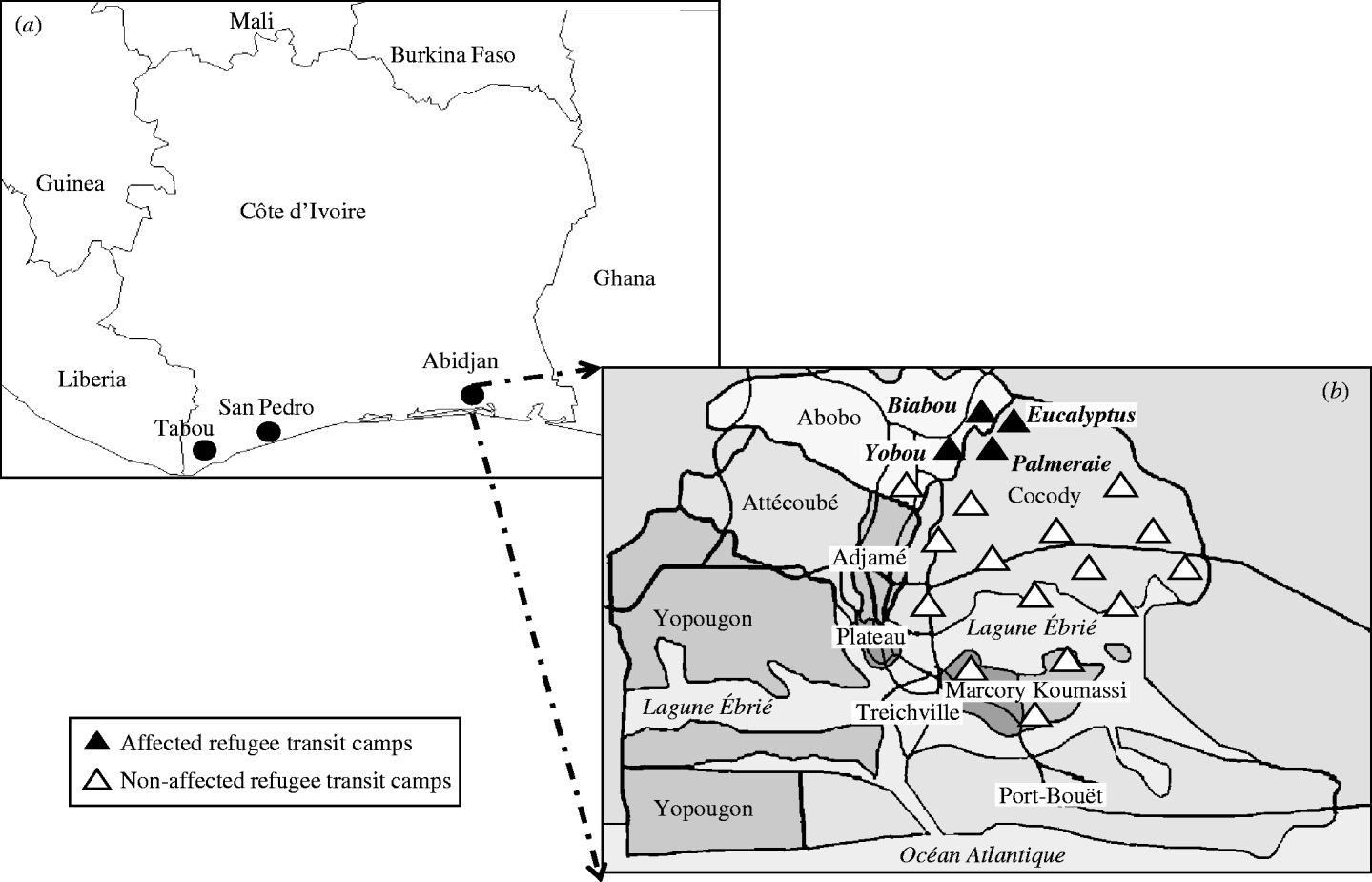
Fig. 1. (a) Map of Côte d'Ivoire: Liberian refugees lived in border-country area of Tabou and San Pedro in large camps or villages before being convoyed to Abidjan transits camps (TCs). (b) Distribution of the 19 TCs hosting Liberian refugees in Abidjan: the four affected TCs (Biabou, Yobou, Eucalyptus, Palmeraie) were located in two municipalities (Abobo and Cocody).
Study and surveillance period
To minimize the potential for the refugees to transmit infectious diseases in camps and during the resettlement process, a DST was established. The aim of the DST was to perform active surveillance in order to detect cases in the early phase and to take timely control action. We documented the results of measles and rubella surveillance from 1 December 2003 (week 1) to 28 June 2004 (week 30). During this period, the DST, consisting of medical doctors and nurses, performed daily surveillance in each TC. Cases were also reported from the health unit established in each camp. A suspect measles case was defined as a refugee, identified by the DST or a camp health unit, who had a generalized maculopapular rash and high-grade fever (⩾38°C). There was no separate clinical case definition to detect rubella. The same case definition for measles was also used to detect rubella cases. An active-case search was conducted family-by-family and each person was checked and screened individually based on family lists. For those people that were absent, follow-up was done over the next days to ensure that there were no unreported cases. A standardized questionnaire was used to collect information from patients, including demographical, epidemiological and clinical information from the camps. If suspect cases of infectious diseases were detected and reported, further investigations were conducted immediately. A meeting was held every day after the surveillance activities to share updates on the situation in the camps, to discuss suspect cases reported, and to make further decisions. Appropriate actions were taken for suspected cases, including isolation, laboratory confirmation, and treatment. When necessary, affected camps were placed in quarantine and scheduled flights for refugee resettlement were postponed. Every case was reported and investigated immediately. All resulting data was kept and maintained in a comprehensive database established by the DST. The information was disseminated during a weekly coordination meeting held at the United Nations High Commissioner for Refugees office in Abidjan and included all partners in the project. This active and comprehensive surveillance system was established and maintained throughout the surveillance period (December 2003 to June 2004).
Laboratory confirmation and case management
Blood specimens from the cases were collected and transported to the Institut Pasteur in Côte d'Ivoire for measles IgM detection by Enzygnost-EIA (Dade Behring, Germany) from 19 January 2004 (week 8). Laboratory testing for rubella IgM (Enzygnost-EIA) and IgG (Microwell-ELISA; Abbott, USA) was reported from 14 February 2004 (week 11). All confirmed cases were monitored and the patients were treated at the TCs or at the health department until they recovered. A measles control and prevention education programme was also provided to families whenever a case was detected. Families were encouraged to adopt certain principles, e.g. bringing sick children to the health facility for treatment, bringing healthy children for immunization and separating sick children from the others in order to reduce the risk of transmission. Mothers were educated to continue feeding and breastfeeding to prevent their children from developing vitamin A deficiency.
Vaccination campaign
In response to the outbreaks, two mass vaccination campaigns targeting children aged between 6 months and 15 years were held at all TCs. The outbreak started from week 8. Once the decision to vaccinate was approved, a vaccination team was established to plan and conduct the vaccination campaign. The team included personnel from the DST, the local health official and health officers from a local NGO. Social mobilization in camps was undertaken by involving the refugees' community leaders in their social activities. The measles vaccination campaign was conducted from 26 February to 4 March 2004 (week 13) and targeted children aged between 6 months and 15 years. A meeting was held every day at the end of the vaccination activities to review progress and address problems arising. The second vaccination campaign was conducted from 13 to 20 April 2004 (week 20) using MMR vaccines.
Data analysis
Demographical and epidemiological data as well as laboratory results were recorded using Microsoft Excel 2000 (Microsoft, USA) and analysed with EpiInfo version 6 (Centers for Disease Control and Prevention, USA) software.
RESULTS
Refugee population characteristics
Biabou and Yobou TCs were the most populated with 1365 (49·3%) and 773 (27·9%) residents, respectively. The majority of the population was male (54·6%). The age range was 0–102 years and the mean age was 23 years. There were 402 (14·4%) children aged <5 years and 37·7% aged <15 years. In total, 60 rash and fever cases were identified during the surveillance period (weeks 1–30).
Measles
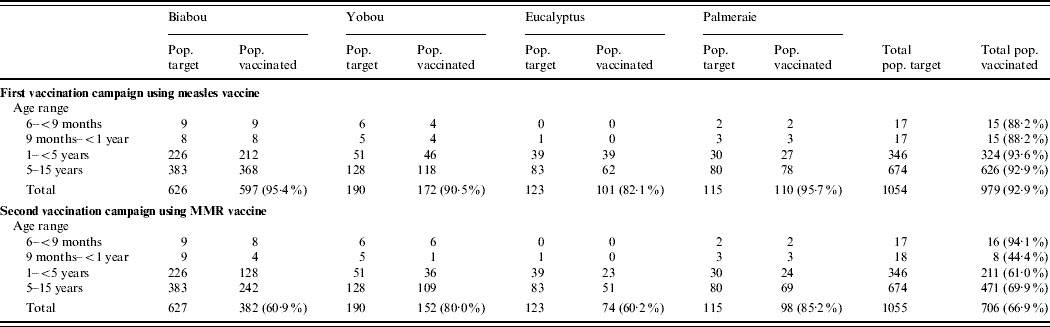
Up to week 13, 11/18 samples (61·1%) tested for measles IgM antibody, were positive. A total of 58 blood samples were tested for measles IgM antibody during the surveillance period, of which 11 (19·0%) were positive (Table 1). The serologically confirmed measles cases were reported from Eucalyptus, Biabou and Yobou TCs with Eucalyptus having the highest incidence rate (4·05%). The mean age for the confirmed measles cases was 3·8 years and ranged from 5 months to 11 years. The highest incidence rate (18·5%) was reported in children aged <9 months (Table 2). The cases included eight females and three males. The measles outbreak lasted for 6 weeks, from 19 January to 23 February (weeks 8–13), without any particular peak (Fig. 2). The first vaccination campaign was conducted from 26 February to 4 March 2004 (week 13), using only the measles vaccine. This vaccination campaign was implemented 6 weeks after the outbreak started. Most (92·9%) of the target population (6 months–15 years) were vaccinated. The vaccination coverage was lowest (88·2%) in the specific group of children aged <9 months (Table 3). Eucalyptus TC had a coverage of 82·1% which was the lowest of the TCs (Table 3). No serologically confirmed measles cases were detected from the remaining suspected cases following this immunization campaign (Fig. 2).
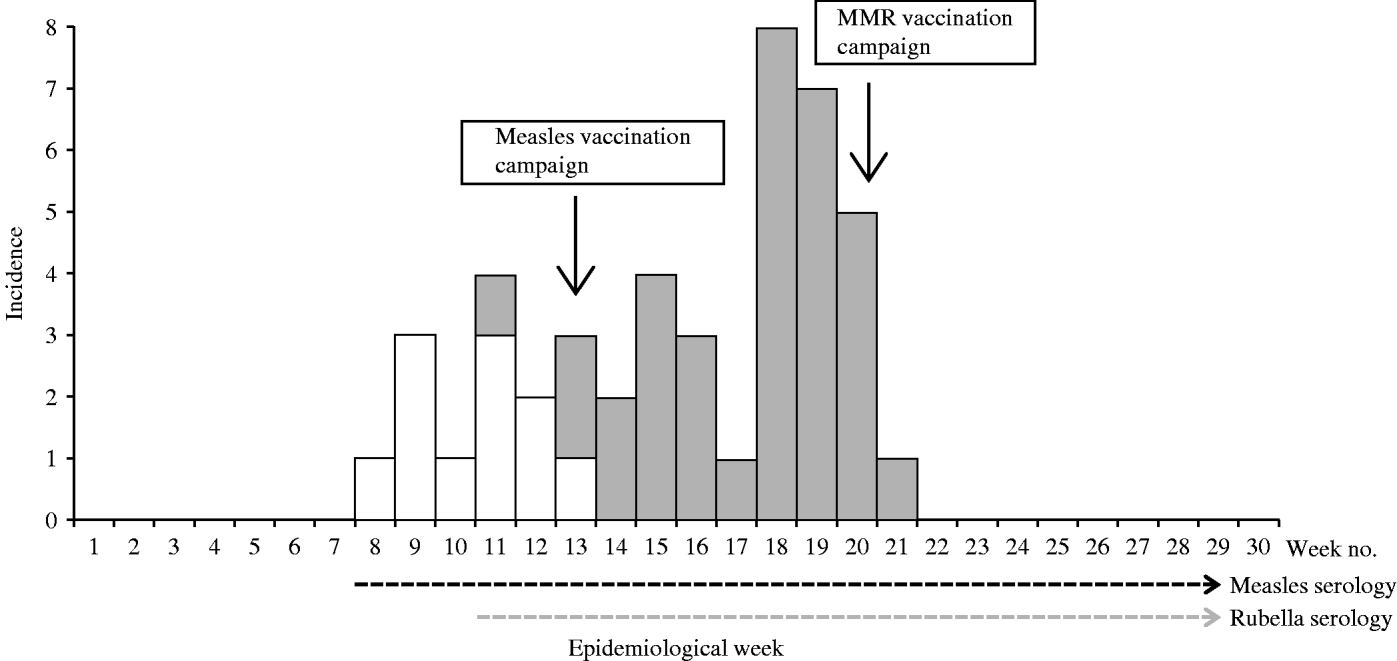
Fig. 2. Weekly evolution of serologically confirmed cases of measles (□) and rubella (![]() ) in the affected transit camps (1 December 2003 to 28 June 2004).
) in the affected transit camps (1 December 2003 to 28 June 2004).
Table 1. Clinical sample details of measles and rubella cases of Liberian refugees in transit camps in Abidjan, 1 December 2003–28 June 2004
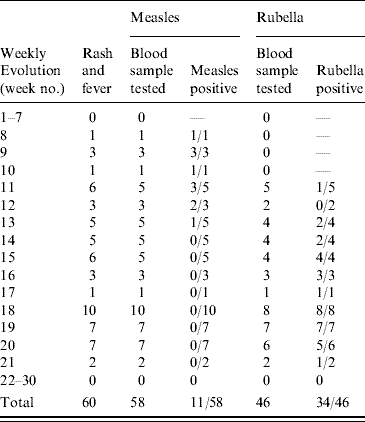
Table 2. Serologically confirmed cases of measles and rubella in Liberian refugees aged <15 years in transit camps in Abidjan, 2003–2004
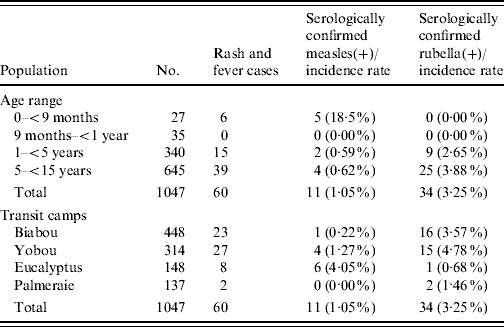
Table 3. Summary of the two mass-vaccination campaigns targeting Liberian children aged 6 months–15 years in transit camps in Abidjan between March and April 2004
Rubella
Up to week 13, 3/11 samples (27·3%) tested for rubella IgM antibody, were positive. A total of 46 samples were tested during the entire surveillance period, of which 34 (74·0%; 18 females, 16 males) were positive (Table 1). Confirmed rubella cases were reported from four camps, Biabou, Yobou, Eucalyptus and Palmeraie (Table 2). The highest incidence rate was reported in Yobou (4·78%). The mean age of confirmed cases was 6·7 years and ranged from 1 to 14 years with the highest incidence rate in children aged between 5 and 14 years of age (Table 2). The rubella outbreak lasted for 11 weeks, from 14 February to 25 April 2005 (weeks 11–21). A peak was observed during week 18 with six cases reported in Yobou (Fig. 2). A supplementary MMR immunization campaign was conducted during week 20 with a reported coverage of 66·9%. The highest coverage (94·1%) was reported for children aged <9 months, and coverage in the Palmeraie camp (85·2%) was the highest (Table 3).
Eucalyptus had reported the highest incidence rate of measles (4·05%), and the lowest incidence rate of rubella (0·68%). From weeks 11–13, both measles and rubella cases were detected in refugees (Fig. 2). No fatality was reported during this investigation.
DISCUSSION
We documented concurrent measles and rubella outbreaks that occurred in 2004 in four TCs for Liberian refugees in Côte d'Ivoire. Measles and rubella are endemic in many countries in sub-Saharan Africa where vaccination coverage is usually low [Reference Riddell, Rota and Rota21–Reference Lawn25]. There are a variety of risk factors for the spread of measles and rubella in displaced populations, which include poor vaccination coverage in countries affected by conflicts [11], international spread of these diseases that occurs during mass migration and high refugee population densities in camps [Reference Gayer19]. During the resettlement process, refugees may spread the measles virus to other international travellers [Reference Rota26] thus facilitating the re-introduction of measles into countries where it had been previously eliminated [Reference Barnett, Christiansen and Figueira27].
This outbreak occurred initially in Liberian refugees who were convoyed into TCs in Abidjan from large camps in border-country areas from where the index case was detected. They were not vaccinated upon arrival at the TCs and had contacted a poorly vaccinated community with unknown vaccination status. Ninety-seven percent of the total rash and fever cases were reported from Biabou, Yobou and Eucalyptus TCs, which continually accepted newly convoyed refugees. A continuous flow of new refugees might facilitate the introduction of these diseases [Reference Kamugisha, Cairn and Akim10]. Existing guidelines advise early vaccination of refugees upon arrival at camps [Reference Oxfam28]. The TCs were located in close proximity to each other and the frequent visits that occurred among them may have also contributed to the increased disease incidence.
WHO reported that MMR vaccine coverage in 2004 was only 49% in Côte d'Ivoire and 42% in Liberia [29]. Camps were located within the city of Abidjan and Liberian refugees had close contact with the neighbouring Ivorian community. Therefore, future interventions should include a simultaneous MMR immunization campaign that is instituted in refugee camps and in surrounding communities as a common effort between international NGOs and local public health authorities. Similar to other studies, most of the measles cases (5/11, 45·5%) were detected in children aged <9 months [Reference Whittle1, 30–Reference Taylor32], suggesting that measles vaccine should be provided for this age group in such populations [12]. Furthermore, a second dose of measles vaccine should be given at age 9 months due to the increased likelihood of primary vaccination failure in younger children in whom maternal antibodies are retained [Reference Aaby33]. This outbreak did not include older measles cases although they have been identified in other investigations [Reference Kamugisha, Cairn and Akim10, Reference Coronado34].
Most of the rubella cases (25/34, 73·5%) occurred in children aged 5–14 years. This contrasts with studies from African countries that report rubella is more likely to occur early in life and that >80% of children are immune by age 10 years [Reference Gomwalk and Ahmad35]. In Turkey, rubella antibodies were present in 38% of children aged 1–4 years [Reference Aksit36]. The preponderance of rubella in young children in this study may be due to the limited exposure to infection of refugees who usually live in remote areas. In many developing countries, as well as in disaster situations, there are a limited number of laboratories that can serologically confirm a disease. Because measles may present with symptoms similar to many other exanthematous diseases (including rubella), and diagnoses are often made based on clinical data alone, there is an increased risk of misdiagnosis. In fact, a high percentage of suspected measles cases are identified as rubella in countries considered to be in a measles-elimination phase [Reference Ramamurty16].
In our investigation, serological testing performed up to week 13 resulted in a higher positive rate for measles IgM (61·1% positive) than for rubella IgM (27·3% positive). As a result, the decision to immunize only against measles was made because the vaccine was readily available and much cheaper than the MMR. Following this immunization campaign, there were no further confirmed measles cases in the remaining patients with rash and fever, but there were an increasing number of confirmed rubella cases. A second vaccination campaign was conducted 2 months later using MMR. Subsequently, there were no further confirmed cases of measles or rubella. If rubella had been serologically confirmed at an earlier stage, together with measles, then the rubella outbreak might have been controlled earlier. This may have led to the appropriate decision to vaccinate initially with MMR in order to terminate the rubella and measles outbreaks. Our study highlights the significance of early laboratory testing for both measles and rubella to confirm the nature of a rash and fever. It also suggests that the MMR vaccine should be given priority in disaster and refugee situations and also in areas with limited laboratory resources. In this case, it may have been more cost-effective to test for measles and rubella earlier, prior to the vaccination campaign [37]. WHO also advise that measles control activities can be used as an opportunity to pursue control of rubella through the use of MR or MMR vaccines [18].
The active and comprehensive surveillance in combination with laboratory testing for measles enabled the detection of the measles outbreak at an early stage. However, the lack of laboratory testing for rubella did not permit early detection of concurrent measles and rubella outbreaks. WHO recommends that coverage >94% is required to prevent an outbreak of measles in displaced populations [12]. In the present study, the measles and rubella outbreaks were successfully controlled after two mass vaccination campaigns targeting children aged from 6 months to 15 years. The outbreak of measles was controlled after the first vaccination campaign with a monovalent measles vaccine (vaccine coverage of 92·9%) while the rubella outbreak was controlled after the second vaccination campaign with a combined MMR vaccine (vaccination coverage of 66·9%). The WHO and UNICEF joint statement for measles mortality reduction in complex emergencies recommends the vaccination of all children aged between 6 months and 14 years plus vitamin A supplementation during emergencies [12]. As a minimum, children aged from 6 months to 4 years must be vaccinated, while vaccine availability, funding, human resources and local epidemiology may influence the target age group [12]. None of the refugees were vaccinated in camps before the outbreak occurred, and the implementation of both vaccination campaigns was delayed. The measles vaccination campaign was implemented 6 weeks after confirmation of the measles outbreak. Trained personnel, vaccines, cold chain equipment and other supplies (syringes, needles, record cards) were not available at the start of the outbreak. There were also a number of administrative and logistical issues to be addressed before the vaccination campaigns began. Even during the campaigns, there were still some obstacles such as lack of cold chain equipment and shortage of vaccines. Measles vaccinations should be given a high priority in emergency relief programmes. Trained personnel, vaccines, cold chain equipment, and other supplies should be available as soon as at-risk individuals begin to gather in camps [Reference Toole38]. Our study indicated that the highest incidence rate of measles was in children aged <9 years (18·5%), but the vaccination coverage (first vaccination campaign) in this specific group was the lowest (88·2%). This is below WHO recommendations. Therefore, based on surveillance data, it will be necessary to prioritize and increase measles vaccination coverage in this age group, particularly in displaced populations. These outbreaks were particularly marked because of the absence of fatalities. This might be due to the active-case-finding approach which functioned quite well by increasing detection, reporting and treating patients at an early stage. Existing studies have documented high case-fatality rates in disaster and refugee settings [Reference Bosan4, Reference Porter6]; in particular, a rate as high as 33% was reported in Ethiopian refugees in Sudanese camps [Reference Shears15]. The involvement of families, community leaders and community health workers from the refugee population, was important in establishing effective surveillance and in mobilizing resources. Moreover, the systematic serological surveillance for both measles and rubella, even though it began late, enabled confirmation of the nature of the second outbreak (i.e. rubella), and implementation of appropriate actions. The present study has presented some interesting aspects of measles and rubella epidemiology in refugee settings and has provided insight for future response and control of outbreaks. However, further research is needed to develop indicators for early warning and surveillance systems in disaster and refugee settings
CONCLUSION
Outbreaks of measles and rubella were detected by the DST in 2003–2004 in Liberian refugees living in overcrowded TCs in Côte d'Ivoire. This active surveillance approach contributed to the successful control of these outbreaks. However, serological testing for both rubella and measles during the early stage of an outbreak and prior to immunization remains critical. Furthermore, it is recommended that vaccination programmes be conducted with MMR rather than with measles vaccine alone, due to the potential misdiagnosis of measles and, for its advantage in the control of rubella and CRS. Routine monitoring of ongoing surveillance data must be considered in order to prioritize camps and to target population age for vaccination strategies in disaster and refugee settings.
ACKNOWLEDGEMENTS
We thank Dr Frank Guttman, Dr Ekissi Alexis, Dr Houffouet Hermann, Dr Kokora Partrice, Bini Jean Claude, Konan Tolla Jean and anonymous persons for the surveillance effort and data collection. We also thank Dr Claude de Ville de Goyet Dr Nobs Roy and Ms Karen Duong for their advice.
DECLARATION OF INTEREST
None.







