INTRODUCTION
Infections with norovirus (NoV) are one of the leading cause of viral gastroenteritis worldwide [Reference Patel1–Reference Hall4]. Typically, infection with NoV is self-limiting and is characterized by nausea, vomiting, abdominal pain and diarrhoea. The incubation period ranges from 24 to 48 h [Reference Karst5]. The most important routes of transmission are faecal–oral, vomit–oral and from person to person. The main vehicle of infection is contaminated food or water. The virus is highly contagious, an estimated dose ⩾18 viral particles is sufficient to cause infection [Reference Teunis6]. Noroviruses are environmentally stable. They can survive freezing, heating (30 min at 60°C) and are resistant to relatively high concentrations of chlorine [Reference Patel1, Reference Koopmans and Duizer7]. Several outbreaks with widespread contamination of environments have been reported, particularly in closed settings [Reference Evans8–Reference Isakbaeva10].
In Finland, the municipal authorities report suspected foodborne and waterborne outbreaks to the national online registry (FWD registry) developed and maintained by the National Institute for Health and Welfare (THL) and the Finnish Food Safety Authority Evira. In 1995, Finland initiated nationwide laboratory-based surveillance for NoV infections. Between 1998 and 2011, the annual number of cases of NoV infection reported to the National Infectious Diseases Register ranged from 125 to 2807. Since 1997, NoV has been the most common cause for foodborne and waterborne outbreaks [11]. During 1998–2002, the most common NoV genogroup causing gastroenteritis outbreaks was genogroup II accounting for 219 (87%) of 252 outbreaks, genogroup I caused 33 (13%) outbreaks [Reference Maunula and Von Bonsdorff12].
On 20 August 2012, an infectious disease nurse from the Epidemiology Unit at the Helsinki City Health Department informed Pirkkala Environmental Health about gastroenteritis in two guests at a wedding buffet that was held on 18 August 2012. The wedding reception took place at a resort/activity centre (hereafter referred to as the centre) in Ylöjärvi, Finland. On 20 August, the municipal authorities were notified of the outbreak through the FWD registry. We investigated the outbreak in order to identify the source and aetiology of the infections, undertake control measures and prevent similar outbreaks in the future.
METHODS
Description of the location
The centre is located on Lake Näsijärvi, in the municipality of Ylöjärvi, in western Finland. The centre consists of a main building with an event hall, kitchen, sauna, 10 guest rooms with toilets, and three cottages.
Epidemiological investigation
NoV was suspected as the cause of the outbreak since the incubation period, and the description and duration of symptoms of the three guests with gastroenteritis were consistent with NoV infection. We defined a case as a person who attended the wedding buffet on 18 August 2012 at the centre and developed at least one of the following symptoms between 18 and 21 August 2012: diarrhoea (⩾3 loose stools a day), vomiting, nausea or abdominal pain.
Health inspectors obtained email addresses of 54 wedding guests and on 30 August, we sent them a web link to a standardized online questionnaire. The self-administered questionnaire gathered information on demographic details, food and beverages consumed during the reception, date and time of onset, duration and characteristic of clinical symptoms, collection of stool specimens and hospitalization.
We performed a descriptive analysis of cases. We compared the exposed with the unexposed through the calculation of attack rates, with 95% confidence intervals also calculated. All statistical analyses were conducted using Stata v. 12 (StataCorp., USA).
Environmental investigation
On 21 August, the municipal health inspectors contacted the wedding organizers, visited the centre, and investigated the general hygiene of the kitchen. The employees of the centre were asked to provide faecal specimens for bacterial and viral analysis and they were supplied with sampling containers. Food samples were not collected since they were no longer available.
Laboratory investigation
Stool specimens were provided by three guests of the wedding buffet with gastrointestinal symptoms. The specimens were tested for Salmonella, Shigella, Campylobacter, Yersinia spp., S. aureus, B. cereus, C. perfringens by routine methods [Reference Murray13] and for NoV using real-time reverse transcription (RT)–PCR assay in a local clinical microbiology laboratory and a virology laboratory, respectively. The specimens of the staff were not tested since the laboratory did not receive a referral.
Water specimens were obtained from the tap in the kitchen, from the lake (on 22 August), and from the ice cube machine (on 4 September). Specimens were tested for gut-derived enterococci, E. coli and coliform bacteria.
On 21 August, the municipal health inspectors collected environmental specimens from the baking board, cutting board and cold pantry handle, to test for aerobic bacteria and Salmonella.
In total, 36 swabs from surfaces at the premises in the centre were taken for NoV analysis. On 22 August, the municipal health inspectors obtained 27 surface specimens for NoV analysis from the main building in the centre. Surfaces were brushed with swabs, which were inserted into a tube containing 5 ml phosphate buffered saline (PBS). The swabs were subjected to nucleic acid extraction using NucliSENS® miniMAG® kit (bioMérieux, The Netherlands) while viral RNA from polymerase-capsid gene junction was amplified using primers and probes specific for NoV genogroups I and II, and QuantiTect probe RT–PCR kit (Qiagen, Germany) in real-time RT–PCR according to the methods previously described [Reference Loisy14, Reference Rönnqvist15]. On 28 August, after the first cleansing, five specimens were taken from places where noroviruses were detected previously. The final collection of four environmental specimens was performed after the second cleansing, on 4 September.
Genotyping analysis was done for three NoV isolates from swabs and from two NoV isolates from patients' stools. Viral RNA was amplified in polymerase region A using a one-step RT–PCR kit (Qiagen) according to Vinjé et al. [Reference Vinjé, Hamidjaja and Sobsey16]. Nucleic acid sequences of the PCR products were determined. A genotyping tool (www.rivm.nl) and BLAST search in Genbank were used for genotype determination. In addition, one NoV isolate from a swab specimen was amplified in region D and used for genotype determination [Reference Vinjé, Hamidjaja and Sobsey16].
RESULTS
Epidemiological investigation
Eighty-eight guests from various countries attended the wedding buffet, of which 54 (61%) had an email address. Thirty-nine (72%) responded to the survey (59% female). The median age of respondents was 37 years (range 27–68 years). Twelve respondents were from abroad (France, n = 6; Italy, n = 4; Switzerland, n = 2). Seven guests had travelled abroad (to France, Italy, Germany, Denmark, Sweden) in the 2 weeks before the wedding reception. Twenty-three (59%) of respondents met the case definition. The highest attack rate (71%) was in the 20–30 years age group. The attack rate was 65% in females and 50% in males (Table 1).
Table 1. Attack rate (AR) of gastrointestinal symptoms by age group and gender, wedding reception, Ylöjärvi, Finland, August 2012
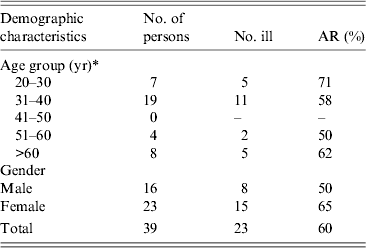
* One person did not report their age.
All 23 cases became ill within a 3-day period, 19–21 August 2012 (Fig. 1). The symptoms of the first case started 12 h after the reception ended. The peak of the outbreak was on 20 August, when 15 (65%) cases fell ill, and the outbreak ended on 21 August.
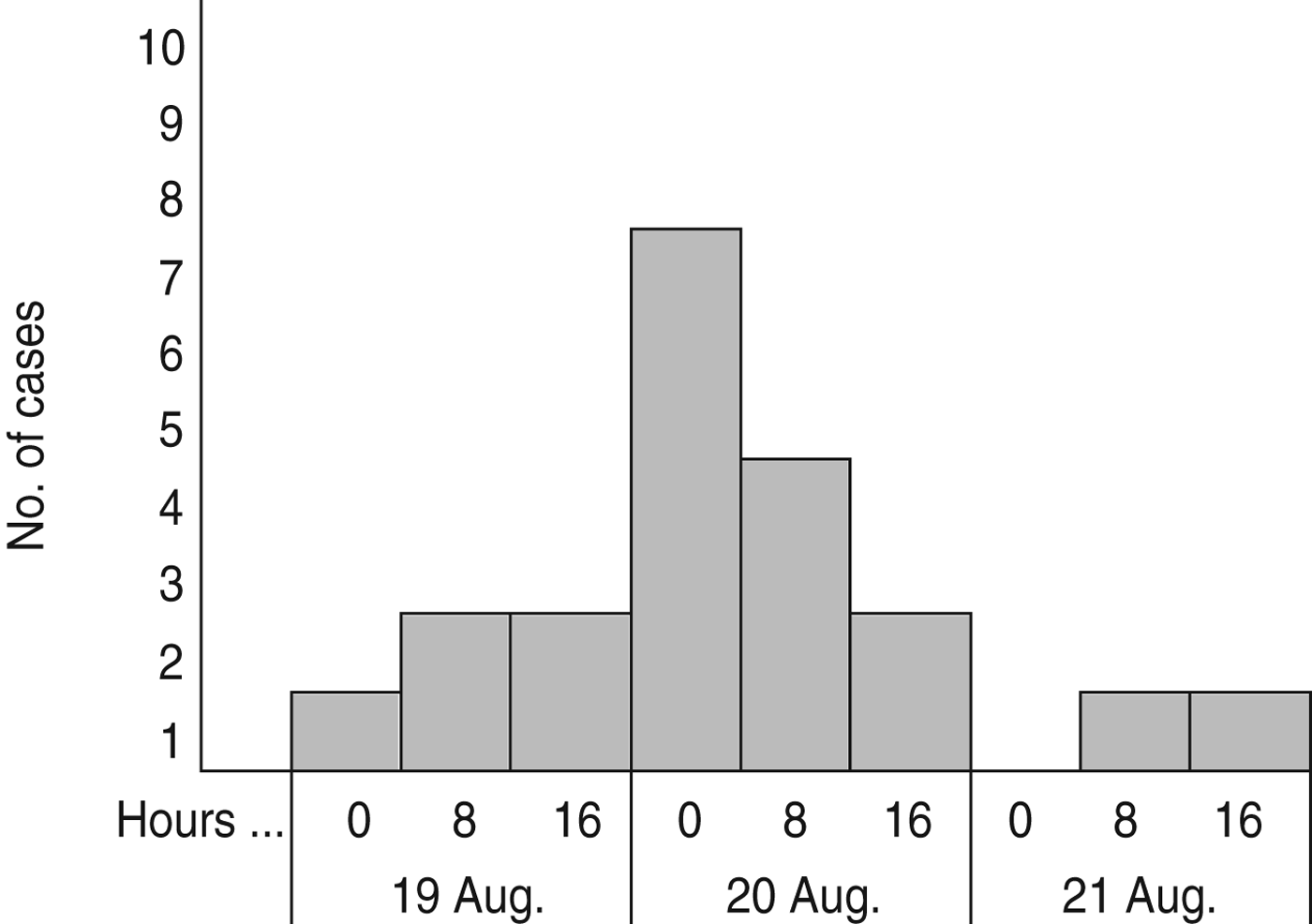
Fig. 1. Distribution of cases with acute gastroenteritis (n = 20) in guests at the wedding reception according to date and time of symptoms onset (three persons did not indicate the time of symptoms onset), Ylöjärvi, Finland, August 2012.
The most commonly reported symptoms were nausea (91%), abdominal pain (74%), diarrhoea (70%), vomiting (48%), headache (39%), and fever (13%). Two cases (9%) were hospitalized.
None of the foods or beverages served at the wedding reception was significantly associated with the illness (Table 2).
Table 2. Attack rates and relative risk of acute gastroenteritis associated with specific food items and beverages consumed during a wedding reception, Ylöjärvi, Finland, August 2012
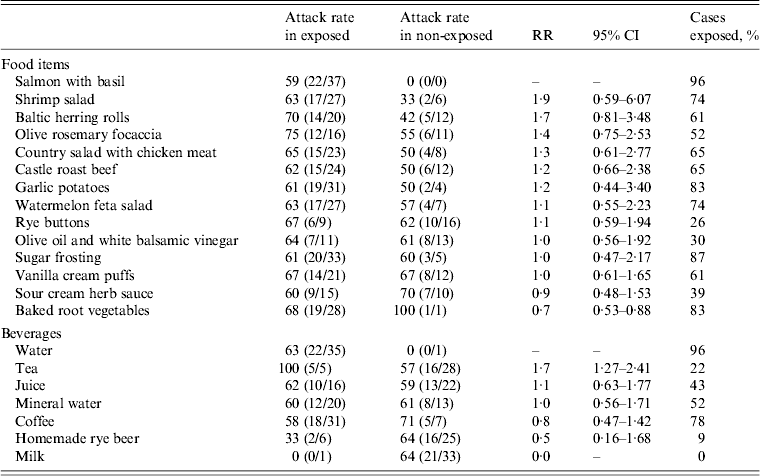
RR, Risk ratio; CI, Confidence interval.
Environmental investigation
In the kitchen, the general hygiene was according to requirements. During the visit to the centre on 21 August and during the phone interview with the manager of the centre on 22 August, the health inspector identified three staff members (two customer service staff and a cleaner) who were ill with symptoms consistent with NoV infection. All had become ill on 21 August, following the wedding, and had not been symptomatic at work. The investigation also indicated that the day before the wedding buffet (17 August 2012), nine people who were staying at the centre, and had received food service, had subsequently developed gastrointestinal illness with symptoms typical for NoV infection after leaving the centre. None of these were accommodated in the same rooms as the wedding guests. Three days after the wedding, the next group of 20 people attended and ate at centre. Within 36 h of their arrival, five persons had fallen ill with gastrointestinal symptoms.
Laboratory investigation
Two stool specimens from the wedding guests were positive for NoV. Salmonella, Shigella, Campylobacter, Yersinia spp., S. aureus, B. cereus, C. perfringens were not found in any of the specimens tested.
Water specimens from the lake and the tap in the kitchen were negative for gut-derived enterococci, E. coli and coliform bacteria (0 MPN/100 ml). The level of heterotrophic spore-forming bacteria [240 colony-forming units (c.f.u.)/ml] in the ice-cube specimen was over the recommended limit (100 c.f.u./ml) of the Finnish Food Safety Authority Evira.
The microbiological quality of the surface hygiene and water specimens collected on 21 and 22 August was satisfactory. The level of aerobic microorganisms was: 76 c.f.u./cm2 for the baking board, 3 c.f.u./cm2 for the cutting board, and 80 c.f.u./cm2 for the cold pantry door handle. All specimens were negative for Salmonella.
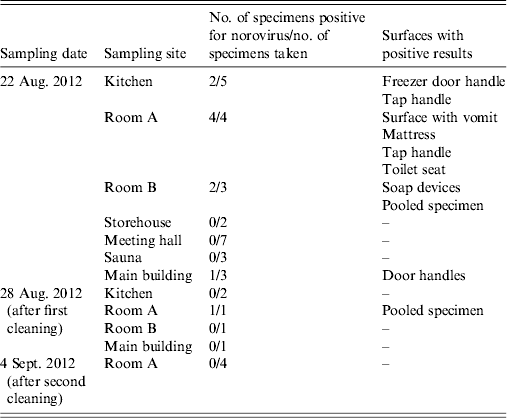
Out of 27 environmental specimens collected on 22 August, nine were positive for NoV. NoV was detected in the kitchen, two hotel rooms and in the main building of the centre (Table 3). The results of five specimens obtained on 28 August taken after cleansing of previously contaminated surfaces, confirmed the presence of NoV in one room. On 4 September, after further cleansing, NoV was no longer detected.
Table 3. Environmental specimens tested for norovirus at the centre, Ylöjärvi, Finland, 2012
NoV isolates from two patients and from three swab specimens taken from the surfaces of one guest room were characterized further by sequence analysis. All sequences were identical and were characterized as NoV genotype GII.4. The virus strain was 98·9% and 98·5% identical with GII.4 Sydney variant (accession no. JX459908) in polymerase region A and capsid region D, respectively.
DISCUSSION
The results of this investigation indicated that the outbreak of NoV gastroenteritis occurred in persons who attended a wedding reception at the centre. NoV was detected in two stool specimens obtained from wedding guests and in 10/36 environmental specimens collected from the centre. The high attack rate and clinical picture reported by the cases are typical for NoV infection. The distribution of cases with a rapid increase and decline and a single peak suggest a point-source outbreak. The statistical analysis of data on food and beverage consumption by the guests did not indicate any specific source of infection. The high attack rate (100%) and risk ratio (1·75) for drinking tea was not considered relevant, since only five cases had been drinking tea at the buffet.
The general hygiene of the centre's kitchen was visually good and none of the kitchen staff had been symptomatic at work. However, NoV was detected on the freezer handle and the kitchen tap handle, indicating that contaminated hands had been touching them. No stool samples from the staff were tested for NoV and none of the food that had been served was available for analysis. In order to assess the microbiological causality, samples from staff members and food served should be available for testing, and the laboratories should be informed about receiving outbreak samples.
Although the microbiological quality of water specimens was consistent with the norm, the level of heterotrophic spore-forming bacteria in the ice-cube machine was over the recommended limit. The investigation indicated that the machine had been out of order for a long time before the wedding buffet and had not been used. It is probable that the number of heterotrophic bacteria in the ice cube machine had increased after the water flow in the machine ceased.
During the control visits the health inspectors identified that several staff members at the centre were ill with gastrointestinal symptoms, although they had not been symptomatic while at work. NoV shedding can continue for several weeks in symptomatic and asymptomatic cases [Reference Tu17, Reference Kirkwood and Streitberg18]. In hotels, where new cohorts of susceptible guests often change, the staff members that excrete the virus, as well as contaminated surfaces, may prolong the outbreak for several weeks [Reference Kuusi19].
The extensive environmental investigation indicated the presence of NoV on several surfaces at the centre. The contaminated surfaces in the main building were easily accessible and commonly used. Since NoV infection has a very low infectious dose and noroviruses were detected on door handles and tap handles, contaminated hands could have played a key role in the environmental transmission cycle. Additional cases of gastroenteritis that occurred before and after the wedding reception supported the hypothesis of an environmental transmission of NoV.
In order to prevent NoV transmission at the centre, we recommended ill staff members stay at home for 2 days after the symptoms had ceased. Careful hand hygiene, always washing with soap and water after toilet visits, and before preparing, serving or eating food decreases the transmission risk [Reference Kuusi, Kanerva and Lyytikäinen20]. In the kitchen, we drew attention to the recommendation of the Finnish Food Safety Authority that 200 g frozen samples of all served foods should be stored for 2 weeks at institutional kitchens to enable microbiological investigations after possible outbreaks.
Since several cases of gastrointestinal illness were reported in three guest groups, the accommodation was left unused for a period of 1 week to cut the environmental transmission cycle. During this period, the accommodation was thoroughly cleansed and disinfected. Control specimens for NoV analyses were taken before the accommodation was returned to use. However, NoV was still isolated in several sites in a hotel room that had been severely contaminated with vomit. The premises were cleansed with detergents and the surfaces disinfected with hypochlorite solution according to THL guidelines [Reference Kuusi, Kanerva and Lyytikäinen20]. We recommend that: contaminated materials should be treated with water and ordinary detergents and disinfected with 1000 ppm hypochlorite or by steam cleaning; materials with vomit or faecal stains can be disinfected with 5000 ppm hypochlorite, with disposable cleaning cloths used. To avoid droplet infection, the use of disposable gloves, eye-nose mask and apron are necessary during cleaning. Closing the centre for 1 week was recommended. After thorough cleansing and disinfection and 1 week's quarantine, no new cases with symptoms typical for NoV infection were identified at the centre.
The sequence analysis of isolates from two patients and three environmental specimens indicated the same genotype in all specimens. The NoV GII.4 Sydney variant was identified. In March 2012, a new variant of GII.4, designated Sydney, was reported in Australia. Since then, increased activity of this variant has been observed worldwide [Reference Van21–23]. The NoV GII.4 Sydney variant was identified for the first time in Finland during this outbreak. It is possible that one of the wedding guests from abroad had imported the new strain to the centre. However, since symptoms typical for NoV infection were also reported in other guests prior to the wedding reception, it is possible that the centre had been contaminated prior to the wedding event. NoV may remain infectious for over 2 weeks on environmental surfaces and in water for over 2 months [Reference Lopman24–Reference Marshall and Bruggink27].
Noroviruses belonging to GII.4 have been the predominant strain worldwide for over a decade [Reference White, Eden and Hansman28]. In 2002, 2004, 2006 and 2010 the number of outbreaks caused by NoV increased markedly [Reference Siebenga29]. Increased NoV activity was associated with the emergence of new variants of GII.4 associated with the multiple outbreaks in the USA and Europe: the Farmington Hills variant in 2001–2002, the Hunter virus at the end of 2004, the GII.4-2006a/2006b viruses in 2006, and New Orleans virus in 2010 [Reference Tu30–33]. In 2012, an increased activity of NoV was observed in Australia, New Zealand, France, and Scotland, which may indicate a new epidemic wave caused by the new variant [Reference Van21].
ACKNOWLEDGEMENTS
We thank Ülle Kärk and Matti Naukkarinen from Pirteva for performing the sampling at the centre; Mrs Alena Kaijalainen and Svetlana Kaijalainen for skilful technical assistance; and Yvan Hutin for valuable comments and suggestions regarding the manuscript.
DECLARATION OF INTEREST
None.






