INTRODUCTION
Respiratory infections are a major healthcare problem in the Inuit population of the Arctic. In Greenland [Reference Koch1], where almost 90 % of the population is of Inuit origin, the incidence of clinically verified upper and lower respiratory infections in children aged 0–2 years is among the highest in the world [Reference Koch1]. In particular, otitis media is highly prevalent, characterized by early onset, recurrent episodes progressing to chronic otitis media with long-term consequences such as hearing loss and impaired language acquisition [Reference Jensen2]. The burden of disease is almost exclusively carried by the Inuit population, whereas non-Inuit in Greenland have lower risks of respiratory infections [Reference Koch1]. In addition, the incidence of invasive pneumococcal disease (IPD) is markedly high in native populations of the Arctic including Greenlandic Inuit with significantly higher mortality than in non-Inuit [Reference Bruce3]. However, reasons for this ethnic health disparity and the high incidence of respiratory tract infections are basically unknown. In other populations, genetic as well as environmental factors including socioeconomic conditions have been shown to influence the risk of respiratory infections [Reference Bruce3]. Except for use of childcare centres, passive smoking, and a parental history of recurrent infections [Reference Koch1], particular risk factors for respiratory tract infections have not been identified in the Inuit.
Worldwide, some of the most clinically relevant bacteria involved in childhood respiratory and invasive bacterial diseases include Streptococcus pneumoniae, non-typable Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus [Reference O'Brien4, Reference Poll, Opal and Van der Poll5]. The bacteria colonize the nasopharynx except for S. aureus which resides in the anterior nasal cavity [Reference Sivaraman, Venkataraman and Cole6]. Nasopharyngeal carriage, which is highly frequent in young children, is considered the essential first step in the development of respiratory and invasive bacterial infections. Although asymptomatic in themselves, the bacteria may migrate to cause either local infections such as otitis media, sinusitis and pneumonia or systemic potentially life-threatening invasive diseases such as bacteraemia and meningitis [Reference Garcia-Rodriguez and Fresnadillo Martinez7]. Nasopharyngeal carriage may thus be considered an infectious reservoir for bacterial autoinfections as well as for transmission to other individuals in the community [Reference Garcia-Rodriguez and Fresnadillo Martinez7, Reference Simell8].
Factors known to influence bacterial carriage rates, besides age, are: bacterial and viral intra- and inter-species interactions (commensal and pathogenic), function of the immune system, and environmental factors [Reference Garcia-Rodriguez and Fresnadillo Martinez7].
In late 2010 the 13-valent pneumococcal conjugate vaccine Prevenar 13® (PCV-13; Pfizer, USA) was introduced in the Greenlandic childhood vaccination programme at ages 3 and 5 months with a booster at age 12 months. The impact of PCV-13 introduction in Greenland is unknown. Its predecessor, the 7-valent pneumococcal conjugate vaccine (PCV-7), which was never used in Greenland, has in other settings been shown not only to reduce incidence of IPD caused by pneumococcal serotypes included in the vaccine (also called vaccine serotypes; VT), but also to prevent nasopharyngeal carriage by VT [Reference O'Brien9, Reference Scott10]. However, invasive disease caused by non-vaccine pneumococcal serotypes (NVT) and other bacteria (so-called ‘replacement disease’) may have emerged as a consequence of the vacant nasopharyngeal niche being refilled by non-vaccine serotype pneumococci or other respiratory pathogens [Reference O'Brien9–Reference Bogaert16]. In 2000, Alaska introduced the pneumococcal vaccine into the childhood vaccination programme (PCV-7). Significant pneumococcal serotype shifts were observed, particularly in the Native Alaskan Inuit population, with reduced prevalence of VT-IPD but also one of the highest reported degrees of IPD caused by NVT and a total incidence of IPD reaching the pre-PCV7 vaccination level [Reference Singleton11].
In this cross-sectional population-based study of Greenlandic children aged 0–6 years, we aimed to determine prevalence and risk factors for nasopharyngeal carriage by S. pneumoniae, non-typable H. influenzae (NTHi), M. catarrhalis and S. aureus, including antibiotic susceptibility testing and patterns of bacterial associations to provide surveillance data during the introduction of PCV-13 vaccine in Greenland.
METHODS AND MATERIALS
Population/study design
Greenland is the world's largest island with more than three quarters covered by ice and a population of 56 370 (2013) persons living in towns and settlements scattered along the coastline. Approximately 88% of the people are Inuit and the rest mainly Caucasians (Danes) [17].
Two towns and nearby settlements were chosen as the study area. Tasiilaq, one of two towns on the East coast with 1800 inhabitants and 800 persons living in three settlements, and Sisimiut on the West coast, the second-largest town of Greenland with 5460 inhabitants and 350 persons in one settlement.
Since 1972, all citizens of Greenland have been given a unique identification number registered in the Civil Registration System (CRS). The daily updated CRS contains vital information on place and date of birth, gender, birth order, siblings, parents, current and earlier addresses [Reference Pedersen18], and the unique personal identification number allows for accurate linkage between other national registers. We identified all children aged 0 to <7 years in October 2011 in the CRS and their parents living in the study area and invited them to participate. After written and oral informed consents were obtained from parents/caregivers, a questionnaire was completed regarding number of siblings, daycare institution attendance, breastfeeding (at any given period and if so duration and if currently breastfeeding), recent antibiotic use (within the last 3 months), domestic tobacco exposure, recent respiratory tract infections (within the last 3 months), hospitalizations (at any given period), number of rooms, number of people sleeping in the same room, in-house water supply and heating source. Data on PCV-13 vaccination status was obtained through nationwide medical files. The study was conducted during autumn 2011, from October to December.
Nasopharyngeal sampling
The standard procedure for nasopharyngeal sampling recommended by World Health Organization in 2003 was used [Reference O'Brien and Nohynek19]. Skim milk-tryptone-glucose-glycerin medium (STGG) has proven useful for the study of respiratory pathogens [Reference Kaijalainen20]. A nasopharyngeal swab sample was taken using minitip flocked nylon swabs (FLOQSwabs™, Copan, Italy) inserted via the nasal cavity into the posterior wall of the nasopharynx, rotated 180° then placed in 1 ml STGG medium and stored at –20 °C for a maximum of 3 weeks before being transported by air at –20 °C to Statens Serum Institut (SSI), Copenhagen, Denmark, for storage at –80 °C.
Laboratory analysis
After thawing, the specimens were vortexed and 50 µl of each sample was streaked onto a 5% horse blood agar, a chocolate agar and an antibiotic chocolate agar plate (SSI). S. pneumoniae, S. aureus and NTHi were primarily targeted on blood agar medium. The cross-streaking with a strain of S. aureus on the blood agar was done to identify NTHi since the two species grow in symbiosis. M. catarrhalis was primarily isolated from the chocolate agar plates. The antibiotic chocolate agar targeted anaerobic species such as Neisseria menigitidis, containing four different antibiotics, polymyxin B (27 246 IE), lincomycin (0·001 g), amphotericin B (0·002 g) and trimethoprim (0·003 g) to inhibit the growth of most Gram-positive and Gram-negative bacteria as well as fungal species. To increase the likelihood of detecting low-density carriage and carriage of multiple pneumococcal serotypes, we also added 50 µl of the nasopharyngeal sample to a 2 ml serum ox broth and incubated in CO2, 37 °C for 24 h, before plating again as described above. This method has proven efficient in increasing the detection level of nasopharyngeal pneumococci [Reference Kaltoft21].
Bacterial identification was based on colony morphology as ascertained by conventional microbiological procedures and verified by MALDI/TOF mass spectrometry [Reference Carbonnelle22]. All isolates were tested for antimicrobial susceptibility using the disk diffusion test and EUCAST breakpoints [23]. Pneumococci were identified based on α-haemolysis, optochin sensitivity and capsular reaction (known as ‘Quellung’). Non-typable pneumococci were identified using the bile solubility test.
Pneumococcal group determination was performed directly on the serum broth-enriched nasopharyngeal samples by Pneumotest Latex® agglutination (SSI, Denmark). Serotypes were identified by Quellung [Reference Merrill24] reaction with the use of type-specific antisera from SSI [Reference Kaltoft21, Reference Slotved25].
Statistical analyses
The use of serum broth enrichment increased the detection of S. pneumoniae, NTHi and S. aureus, whereas detection of M. catarrhalis was reduced when using this enrichment. Due to these selective growth advantages, we decided to base the analysis of carriage rates on either a positive original or serum broth-enriched nasopharyngeal sample. However, since serum broth addition changed the bacterial composition, the analyses of risk-factor and bacterial associations were based solely on results from the original swab samples.
Risk-factor analyses and tests for inter-bacterial associations were done by logistic regression analysis (proc logistic; SAS v. 9.3, USA) with bacterial colonization as outcome. Each exposure variable was first tested separately in a univariable model and only if significant at the 5% level included in a multivariable model. Based on this, the final model was adjusted for age, sex, ethnicity and PCV-13 status (i.e. having received ⩾1 dose of PCV-13 or not). Pneumococcal serotypes were grouped in vaccine types (VT), i.e. serotypes included in the PCV-13 vaccine and non-vaccine types (NVT), not included in the PCV-13 vaccine, and treated as separate groups of bacteria in the analyses.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the Greenlandic Scientific Commission (Journal no. 2011–056257, doc. no. 738293) and the Danish Data Protection Agency (2008-54-0427).
RESULTS
A total of 450 children were invited and 352 (78%) children aged 0–6 years [median 2·8 years, interquartile range (IQR) 1·1–4·8 years] consented and were enrolled. The majority (92%) was of Inuit origin and 52% were males (Table 1). Approximately one third of the children had received ⩾1 dose of PCV-13 vaccination (13% one dose, 16% two doses, 8% three doses). The median age of children who had received ⩾1 dose of PCV-13 was 1·2 years (IQR 0·6–1·7 years) and the median age of unvaccinated children was 4·4 years (IQR 3–5·6 years).
Table 1. Demographic characteristics of 352 healthy Greenlandic children aged 0–6 years, 2011, Tasiilaq and Sisimiut, Greenland
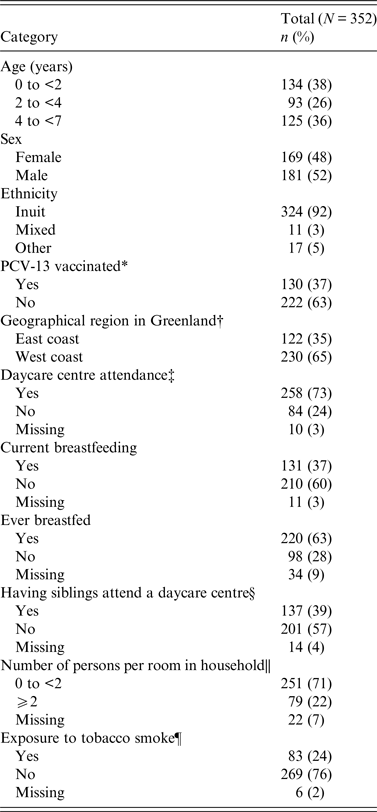
* PCV-13: Having received ⩾1 dose of the 13-valent pneumococcal conjugate vaccine or not.
† Geographical site: East Greenland (Tasiilaq) and West Greenland (Sisimiut).
‡ Daycare attendance: Current attendance at a daycare centre.
§ Sibling in daycare: Having siblings attend a daycare institution.
|| Persons per room: Number of persons per household divided by number of rooms exclusive of kitchen, entrance hall and bathroom.
¶ Tobacco exposure: When one or both parents/caregivers smoke.
The serum broth enrichment increased the detection of low-density growth, in particular S. pneumoniae, NTHi and S. aureus, whereas the detection of M. catarrhalis was reduced (Table 2).
Table 2. Overall nasopharyngeal carriage rates in 352 healthy Greenlandic children aged 0–6 years. Results listed according to positive culture from either the original nasopharyngeal samples, the serum broth-enriched samples, positive in samples either with or without serum broth enrichment and finally positive in both types of samples
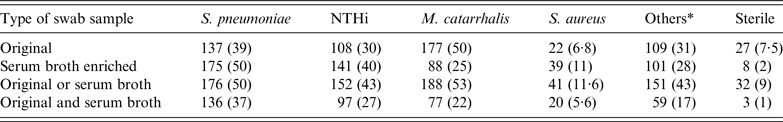
NTHi, non-typable Haemophilus influenza.
Values given are n (%).
* Others: Moraxella species in particular nonliquefaciens, coagulase-negative staphylococci, non-haemolytic streptococci, Haemophilus influenzae types B, E and F (n = 5, 1%) and haemolytic streptococci groups A, B and G (n = 9, 2·5%).
Carriage of potentially pathogenic bacteria
Overall, 293 (83%) children carried one or more potentially pathogenic bacteria (Fig. 1 a). Carriage was established at an early age with 85% of infants aged <2 months being colonized, rising to 100% in children aged 2–4 months. Carriage rates fluctuated between 85% and 100% during the first year (Fig. 1 a). Thereafter, a steady decline to reach an overall carriage rate of 70% in 6-year-olds was observed (Fig. 1 b).

Fig. 1. (a) Nasopharyngeal bacterial carriage in healthy Greenlandic children aged (a) 0–12 months, (b) 0–6 years.
M. catarrhalis
The most frequent colonizing bacteria was M. catarrhalis with an overall carriage rate of 53% (188 isolates). Rates increased during the first year and peaked in those aged 1–2 years (70%), and then decreased to 20 % in preschool children. All isolates were resistant to penicillin and ampicillin, intermediately resistant to cephalosporins (cefuroxime) and fully susceptible to macrolides.
S. pneumoniae
A total of 185 S. pneumoniae isolates were detected in 178 children, due to co-colonization with multiple serotypes in 4% of participants, resulting in an overall carriage rate of 50%, with the highest carriage rate before age 3 years (60%) levelling off to 45% in subsequent years (Fig. 1 b). Carriage was detected as early as age 2 weeks, and by age 2 months 50% of children carried pneumococci (Fig. 1 a). The most frequently carried serotypes were 6B, 6C, 15B, 16 F, 19A, 22 F, 23 F, 33 F and 35 F accounting for 78% of all isolates. Of these, only serotypes 6B and 19A are included in the PCV-13 vaccine. All S. pneumoniae isolates were fully susceptible to penicillin (oxacillin) except one isolate of type 6C that also showed resistance to erythromycin and clindamycin.
NTHi
Overall carriage was 43% (152 isolates), with increasing rates during the first 2 years from 10% to 60%. After peaking in 2-year-olds rates dropped to 35% in children aged 5–6 years. We only identified three capsular H. influenzae isolates (types B and E). Of NTHi isolates, 36% were penicillin-resistant and β-lactamase-producing and thus resistant to ampicillin, amoxicillin and piperacillin according to EUCAST breakpoints v. 3·1 [23]. Five percent of NTHi isolates were resistant to trimethoprim-sulfamethoxazole.
S. aureus
A different carriage pattern was observed for S. aureus with highest rates in children aged 0–1 month (50%), followed by a decline to around 10% in 1-year-olds where it stabilized until age 6–7 years. A total of 41 (11·6%) isolates were identified. The majority (90%) of S. aureus isolates were penicillin-resistant but susceptible to dicloxacillin.
Risk factors for carriage
For all pathogens young age was significantly associated with increased bacterial carriage (Table 3). Crowding-related factors such as attending a daycare institution, having a sibling in daycare or living with ⩾2 persons per room, increased the odds of bacterial carriage (except for S. aureus). Furthermore, we found significantly higher carriage rates of NTHi and M. catarrhalis in children from Sisimiut compared to children from Tasiilaq even when adjusting for daycare attendance. Ethnicity did not show a clear association with carriage. Neither did breastfeeding, recent antibiotic use, recent respiratory tract infections, hospitalizations, in-house water supply and heating source (data not shown).
Table 3. Risk factors for carriage of S. pneumoniae, non-typable Haemophilus influenzae, M. catarrhalis, S. aureus or ⩾1 of either bacteria in Greenlandic children aged 0 to <7 years in Tasiilaq and Sisimiut 2011, adjusted for age, sex, ethnicity and PCV-13 status

OR, Odds ratio; CI, confidence interval; NTHi, non-typable Haemophilus influenzae; PCV-13 status, 13-valent pneumococcal conjugate vaccine.
* PCV-13: having received ⩾1 dose of PCV-13 or not.
† Region: East Greenland (Tasiilaq) and West Greenland (Sisimiut).
‡ DC attendance: Current attendance at a daycare centre. Missing: 10 (3%).
§ Sibling in daycare: Having siblings attend a daycare institution. Missing: 14 (4%).
|| Person per room: Number of persons per household divided by number of room exclusive kitchen, entrance hall and bath. Missing: 22 (7%).
¶ Tobacco exposure: One or both parents/caregivers smoke. Missing: 6 (2%).
Significant findings (P < 0·05) in bold.
Bacterial associations
Co-colonization between any two or more of the studied bacteria appeared in 52% of children, primarily in young infants, and most frequently between S. pneumoniae and M. catarrhalis (33%). NTHi showed a positive association with NVT pneumococci and M. catarrhalis, respectively, but a negative association with S. aureus (Table 4). M. catarrhalis was positively associated with S. pneumoniae, particular VT pneumococci, and negatively associated with S. aureus. When restricting the pairwise interaction analysis to individuals with no concurrent colonization by any other of the studied bacteria none of the estimates changed in direction, but some interactions were less pronounced. However, the association between NTHi and M. catarrhalis, remained positive (odds ratio 4·0, 95% confidence interval 1·3–11·7).
Table 4. Odds of co-colonization between pneumococcal vaccine (VT) or non-vaccine (NVT) serotypes, non-typable H. influenzae (NTHi), M. catarrhalis and S. aureus in 352 Greenlandic children aged <7 years. Analyses are based on isolates from original swab samples without serum broth enrichment and with no restrictions regarding other potential co-colonizing bacteria. OR are adjusted for age, sex, ethnicity and PCV-13 status

aOR, Adjusted odds ratio; NTHi: non-typable Haemophilus influenza.
* VT, Pneumococcal serotypes included in the 13-valent pneumococcal conjugate vaccine (PCV-13).
† NVT, Pneumococcal serotypes not included in PCV-13 vaccine.
Significant findings (P < 0·05) in bold.
DISCUSSION
Overall, we found nasopharyngeal bacterial colonization in Greenlandic children to occur unusually early in life, with frequent bacterial co-colonization and continuing high carriage rates in older children. Furthermore, we found crowding-related risk factors for bacterial carriage and indications of clinically important associations between four key pathogens frequently related to respiratory and invasive infections in young children.
Pneumococcal carriage was acquired within the first 2 weeks of life and followed by a prevalence of about 60% during the next 2 years. In a systematic review of nasopharyngeal carriage in children, Adegbola et al. found that pneumococcal carriage rates in children from low- and lower-middle-income countries generally were higher (up to 93%) compared to middle- and high-income countries where the highest reported carriage rates were 58% [Reference Adegbola26]. High carriage rates of pneumococci have been associated with a high prevalence of respiratory infections [Reference Garcia-Rodriguez27]. Greenland has one of the highest incidences of respiratory tract infections [Reference Koch28]. Furthermore, invasive pneumococcal disease is frequent in Inuit in the Arctic [Reference Bruce3] and S. pneumoniae is the most frequent cause of invasive bacterial diseases in Greenland, associated with high morbidity and mortality [Reference Meyer29]. Yet, unlike most other high-risk populations with very high rates of bacterial carriage [Reference Mackenzie30–Reference Van Den Biggelaar32], this population-based study shows carriage rates comparable to those of paediatric populations from Europe and the United States with much lower incidences of respiratory tract infections [Reference Garcia-Rodriguez27, Reference Harboe33]. In other words, the carriage rate in itself does not seem to be the most significant feature for the high disease burden in Greenlandic children. However, we observed that carriage in our population occurred very early, i.e. at age 2 weeks, compared to a mean age of 6 months for first acquisition in paediatric populations from Europe and the United States [Reference Garcia-Rodriguez and Fresnadillo Martinez7]. Furthermore, we saw a high rate of co-colonization in our population, which is likely to have implications for polymicrobial respiratory infections. These findings correspond to findings in other high-risk groups, although relatively few studies have described the combined carriage of respiratory pathogens. In a carriage study of Australian Aboriginals aged 0–2 years, who share the same very high disease burden from otitis media as the Greenlandic Inuit, they found higher carriage rates of respiratory pathogens and more frequent carriage by multiple pathogens in Aboriginal children compared to non-Aboriginals and colonization in Aboriginals began at an earlier age. The numbers were compatible with our findings [Reference Mackenzie30]. Furthermore, in the Australian study as well as in the present study, the overall carriage rates were only moderately reduced with age as opposed to low-risk populations where the rates after a peak incidence in 2-year-olds decreases gradually [Reference Simell8]. This pattern of early age at first acquisition and ongoing polymicrobial colonization through infancy and childhood may account for the high disease burden in this Inuit population.
To our knowledge, previous studies on bacterial nasopharyngeal carriage in the Inuit population, have almost exclusively focused on pneumococcal carriage without including other potential pathogenic bacteria [Reference Moore34, Reference Hammitt35]. Only a single Greenlandic study from 1993 has described nasopharyngeal bacterial and viral co-carriage in young children with acute otitis media and healthy controls. However, in that study, few risk factors for carriage of specific bacteria were identified, except for young age and current acute otitis media that were associated with increased S. pneumoniae carriage rates [Reference Homøe36].
We consistently found that young age increased the odds of bacterial carriage regardless of the studied species. This association has partly been attributed to close contacts and high transmission rates between young children combined with poorly developed immunity in this age group. In line with other studies [Reference Principi37] breastfeeding did not seem to protect against bacterial carriage. Breastfeeding has, however, been shown to lower the risk of otitis media and invasive pneumococcal disease [Reference Garcia-Rodriguez27, Reference Levine38]. This could indicate, that higher levels of serotype-specific serum IgG than what is achievable from breastfeeding may be required to prevent or clear colonization. As suspected, crowding-related factors increased the risk of carriage. Attending a daycare institution is quite common in Greenland with 73% of the children in this study doing so. Furthermore, the average number of persons per household is higher in Greenland (3·4 in towns and 4·4 in settlements) than in, for example, Denmark (overall 2·1) [17]. Ethnicity in itself did not show any association with carriage suggesting that environmental factors play the most critical role for the risk of nasopharyngeal carriage in this population. However, the number of non-Inuit was low. Hence, the study may be underpowered for conclusions regarding ethnic associations. This study was conducted during autumn. The effect of seasonality on nasopharyngeal bacterial carriage is inconsistent and seems to vary according to the study population. Midwinter has been associated with an increased rate of carriage for reasons that remain unclear, but it may be related to closer interpersonal contacts due to more indoor activity. However, in other studies the effect of season is absent [Reference Garcia-Rodriguez and Fresnadillo Martinez7]. Homøe et al.'s [Reference Homøe36] study on nasopharyngeal carriage in Greenlandic children did not study potential seasonal variation on rates of carriage, but Koch et al. found no seasonal pattern of the overall incidence of acute respiratory infections [Reference Koch28] during a 2-year cohort study of Inuit children living in Greenland.
Odds for NTHi carriage were substantially increased if NVT pneumococci or M. catarrhalis were also detected. Diverging results have been reported on co-colonization with these pathogens [Reference Dunne39]. Xu et al., who studied both healthy children and children with an upper respiratory infection noted that bacterial interactions differed in healthy and symptomatic children. This may indicate that inter-bacterial dynamics are influenced by infections [Reference Xu40].
The positive association between NTHi and NVT pneumococci, but not VT, may point towards a serotype-specific association between NTHi and pneumococcal serotypes. Spijkerman et al. [Reference Spijkerman14] found persistently higher prevalence rates of NTHi carriage and pneumococcal non-PCV-7 types in young asymptomatic children 3 and 4½ years after PCV-7 vaccination together with an almost complete eradication of PCV-7 type pneumococci.
The Finnish Otitis Media trials found an increase in the proportion of otitis media caused by NTHi following PCV-7 vaccination compared to the pre-PCV-7 era [Reference Eskola41]. This raises the question if increased NTHi carriage rates can be expected after widespread use of PCV-13 in Greenland due to the inter-bacterial interaction and the anticipated serotype shift from VT- to NVT-colonizing pneumococci. Theoretically, that may put PCV-13-immunized children at a higher risk of otitis media caused by NTHi.
This study has some limitations. Since we only recruited children from two areas of Greenland we cannot be certain that they are representative of the whole country. Moreover, the study was conducted during the first year after the introduction of the PCV-13 vaccine and thus the observed carriage rates may to some extent be confounded by this vaccine introduction. However, this effect is likely to be limited for various reasons. First, only 37% of the children had received ⩾1 PCV-13 vaccination, second only 8% of the children were fully vaccinated with three doses, and finally vaccination only occurred in the youngest children. The inter-bacterial associations we have observed may be temporary during the introduction of PCV-13. Whether these associations persist may be clarified by a follow-up study that recently has been carried out in the same study area. Finally, the association models between two species may be simplistic and in fact not reflect the complexity of events occurring within the nasopharyngeal flora, and thus only be revealed in studies using more complex methods, such as microbiome studies.
In conclusion, we found the overall nasopharyngeal carriage rates in healthy children in Greenland to be comparable with low-risk paediatric populations, although colonization begins very early in life, with frequent bacterial co-colonization, and ongoing high carriage rates of four clinically relevant bacteria. Our results also indicate that colonization does not occur at random but may be affected by important inter-bacterial associations.
ACKNOWLEDGEMENTS
The authors give special thanks for the help and hospitality provided by the chief medical doctor at the Hospital of Tasiilaq, Hans-Christian Florian Sørensen and at the Hospital of Sisimiut, Ove Rosing Olsen, as well as the staff at the hospitals helping with the logistics. The fieldwork would not have been successful without the extensive help from the interpreters Susanne Vid Stein and Antoinette Kuitse. A huge effort was made to finish the analysis of nasopharyngeal samples in time by the technical laboratory staff at the Statens Serum Institut, with special thanks to Kirsten Olsson, Kirsten Burmeister and Monja Hammer as well as medical student Jacqueline Mistry. We are sincerely grateful to the many participating children and their families for spending their time with us and giving us the opportunity to fulfil the study. Finally, we are very appreciative of the friendly help from the public schools and daycare institutions of Sisimiut and Tasiilaq.
The study received funding from The Commission for Scientific Research in Greenland, co-financed by The Danish Research Council (grant no. 10–0905576); The A. P. Møller Foundation for the Advancement of Medical Science; and the Aase & Ejnar Danielsens Foundation.
DECLARATION OF INTEREST
H. C. Slotved participates in a research project supported by Pfizer and declares no conflict of interest regarding the present study. None of the other authors have any conflict of interest.








