INTRODUCTION
The majority of paediatric HIV infections are the result of mother-to-child transmission (MTCT), which can take place in utero, during labour and delivery and also postnatally through breastfeeding. MTCT rates without any intervention prior to the introduction of the highly active antiretroviral therapy (HAART) were as high as 15–25% in Europe and 16–30% in the USA, and higher rates were reported from Africa [1]. Women who are at greater risk of vertical transition include patients with symptomatic HIV infection, those who undergo primary infection, suffer from severe immunosuppression or having lower CD4 count and higher viral load, especially in the genital fluids [Reference Ioannidis2]. Maternal characteristics, such as older age and the use of illicit drugs, have been associated with perinatal HIV transmission [Reference Mofenson3]. Additionally, vaginal delivery (vs. caesarean section), prolonged duration of rupture of membrane (PROM) and possibly invasive obstetric procedures might also increase transmission risks [Reference Thorne and Newel4]. Breastfeeding, especially when performed non-exclusively, is associated with a higher risk of MTCT [Reference Iliff5], and mothers are therefore recommended to use formula feeding.
HAART has been available in Israel since 1996 at no charge for citizens, and become the standard of care for HIV-infected pregnant women. HAART improves maternal health condition and decreases the risk of MTCT. Neonates are usually treated with 6 weeks of antiretroviral (ART) prophylaxis with zidovudine syrup in most cases following the delivery, with avoidance of breastfeeding, to decrease the risk of vertical transmission. It has been reported that these interventions in developed countries have reduced MTCT rates to 1–2% [6].
Israel is a developed country with a gross domestic product of US$ 32 600 per person, including nearly 8·1 million citizens, of whom nearly 2·5 million (~31%) are non-Israeli born, and about 200 000 (~2·5%) are migrants who are non-citizens who mostly arrived in Israel mainly for labour purposes [7]. HIV/AIDS burden in Israel is relatively low, and the incidence in 2010 was 5·6 diagnoses in 100 000 population. However, individuals from key risk-groups, such as migrants originated in high HIV prevalence countries and intravenous drug users were disproportionally high (HIV point prevalence rates in 2010 were 1805 and 3150 for 100 000 population, respectively) [Reference Mor8].
The aims of this study were to describe temporal vertical transmission rates in Israel and analyse the changes over time in relation to HAART introduction in 1996.
METHODS
This historical prospective study included all HIV-infected women who delivered in Israel and were local citizens between January 1988 and December 2011. The women who were notified to the National HIV/AIDS Registry (NHAR) in the Ministry of Health [Reference Mor8] were crossed-matched with the civil census to obtain the list of children born to each woman and verify their survival by December 2011. Infants who were born to non-citizen mothers were excluded, as they were not registered in the civil census, thus cross-matching was not possible. We also excluded children who were born before the mother migrated to Israel. All children who were born in Israel to HIV-infected Israeli female citizens were then cross-matched with the NHAR to identify if they were infected vertically.
The medical charts of both the mother and infants from the different hospitals were reviewed to obtain information on demographic characteristics, prenatal care and HAART including adherence during pregnancy and labour, postnatal ART prophylaxis to the newborn, breastfeeding habits, gynaecological history and gestational age, latest maternal CD4 count and viral load, when available.
Maternal HAART regimen was defined as three drugs, typically including two nucleoside reverse transcriptase inhibitors with either a protease inhibitor or non-nucleoside reverse transcriptase inhibitors. HAART adherence among pregnant women was classified to complete or inadequate according to the notes registered in the medical records. Mono or dual ART during pregnancy was considered as partial therapy. Late maternal diagnosis was determined as 1 month or less prior to delivery. Infants were categorised as being HIV infected if they had a positive PCR test prior to 18 months of age or a positive HIV antibody test at 18 months of age or later. Breastfeeding habits were determined at the discharge from the hospital after delivery according to the hospitals’ records. Maternal prior tuberculosis infection was identified by cross-matching the NHAR with the National Tuberculosis Registry.
Maternal demographic characteristics, gynaecological details, pregnancies’ outcomes, HAART treatment and infant care were compared between newborns who were infected vertically with those who did not acquire the infection, and also between infants who were born before 1996 when HAART were introduced with those who were born after 1997. Categorical variables were compared by the χ 2 tests or the Fisher's Exact test when expected cell size was <5. Continuous variables were compared by the Student's t-test in case they distributed normally and by the non-parametric Mann–Whitney test in all other cases. All variables which achieved P-values <5% in the univariate analysis were considered statistically significant and were included in the multivariate analysis in the logistic regression model to identify variables which may predict vertical transmission, after considering collinearity, yielding odds ratios and 95% confidence intervals (CI). Changes in time trends were tested using piecewise linear regression analysis. Statistical analyses were performed using SPSS software for Windows, Version 20·0, Chicago, IL. The study was approved by the Institutional Review Board of the E. Wolfson Hospital.
RESULTS
During the study period, 796 infants were born to HIV-infected women, of whom 25 (3·1%) acquired the infection, in declining trends during the study period, P < 0·01 (Fig. 1). Of all 80 infants who were born prior to the introduction of HAART, 13 (16·3%) were infected vertically, while of 716 who were born after 1997, 12 (1·7%) acquired the infection, P < 0·01. Women who transmitted the infections vertically were more likely to be younger than mothers who did not transmit the infection (Table 1). They were also more likely to deliver trans-vaginally, less likely to be treated with HAART during pregnancy, delivery or labour and gave birth before 1996. Newborns who were not treated postnatally with ART prophylaxis were more likely to be infected and consequently their mortality rate was higher. All HIV-infected infants who died were <3 years of age. In multivariate model, the uptake of maternal HAART during pregnancy and infant ART prophylaxis were two variables which predicted low risk of vertical transmissions (Table 2).

Fig. 1. Number of infants born to HIV-infected mothers in Israel and rates of vertical transmission, 1988–2011.
Table 1. Maternal and newborn characteristics, by vertical transmission
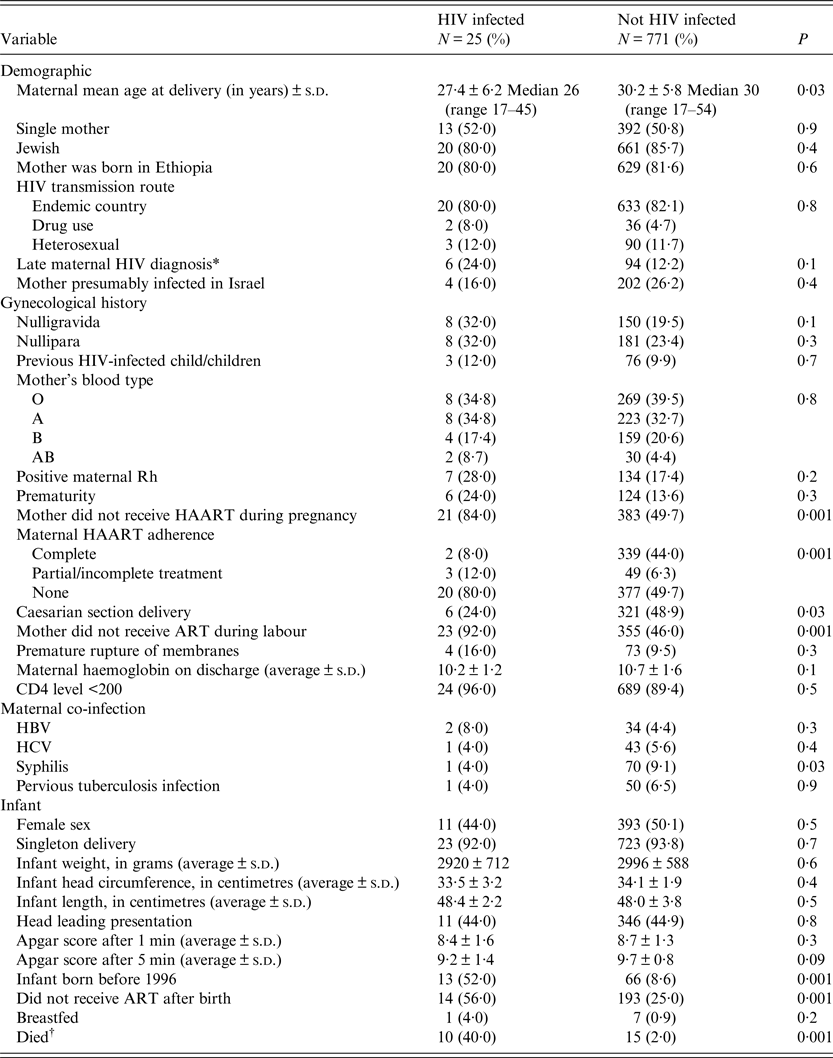
s.d., standard deviation; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
* Less than 1 month before labour.
† Adjusted to the year of infant birth and to age during death.
Table 2. Adjusted odds ratio for vertical transmission of HIV
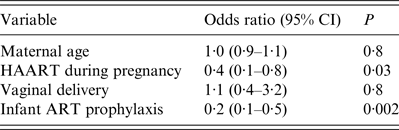
ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
Pregnant women who delivered before 1996 (Table 3) were younger than those who gave birth after the introduction of HAART, more likely to be singles and Jewish. Their infection was detected late during the pregnancy, more likely to be nulliparous and null gravida, delivered trans-vaginally, and less likely to receive HAART during pregnancy, labour and delivery. Their CD4 levels were lower and they were more likely to be co-infected with HCV and previously diagnosed with tuberculosis. Infants who were born before 1996 had higher birth weight, were less likely to be situated in head presentation, to receive ART prophylaxis and their mortality rate were higher compared with those born after 1997.
Table 3. Maternal and newborn characteristics, by period of delivery
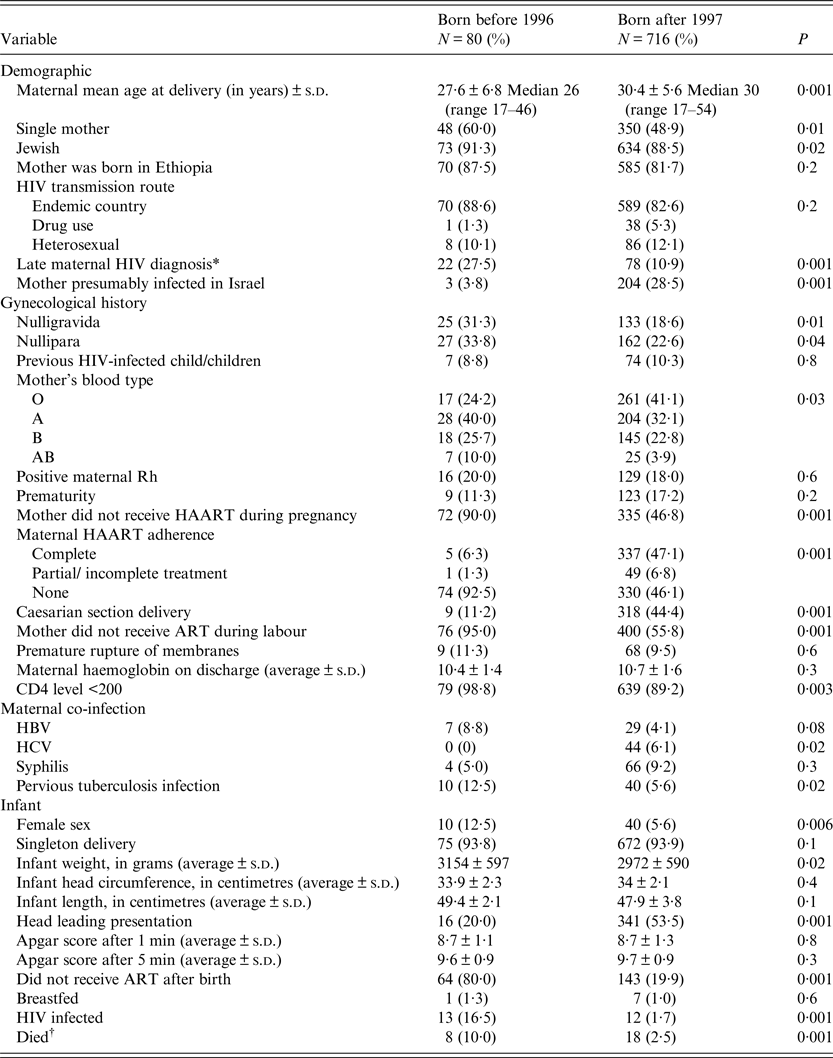
s.d., standard deviation; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
* Less than 1 month before labour.
† Adjusted to the year of infant birth and to age during death.
The rate of caesarean section increased from 12·7% among pregnant women who delivered before 1996 to 30·0% among pregnant women who delivered after the introduction of the HAART, P < 0·02. The overall vertical HIV transmission rate associated with vaginal deliveries was 4·6%, vs. 1·8% of the infants who had been delivered by caesarean section, P < 0·01, while among mothers who received HAART the respective transmission rates were 1·5% and 0·6%, P = 0·6.
Of 342 pregnant women who received HAART during pregnancy and adhered to the treatment, only one (0·3%) newborn acquired the infection (Table 4). In this case, the mother did not receive ART during labour and the infant was not treated postnatally. Of the 45 pregnant women who received antepartum HAART and their adherence was incomplete, two (4·5%) transmitted the infection. These mothers were not treated during labour and one infant did not get ART prophylaxis. Of the 409 pregnant women who did not use HAART during pregnancy, 22 (5·3%) infants acquired the infection, mostly if HAART treatment was neither provided to the mothers at labour nor ART to the newborns after delivery.
Table 4. Rates of vertical transmission by HAART during pregnancy and labour and breastfeeding
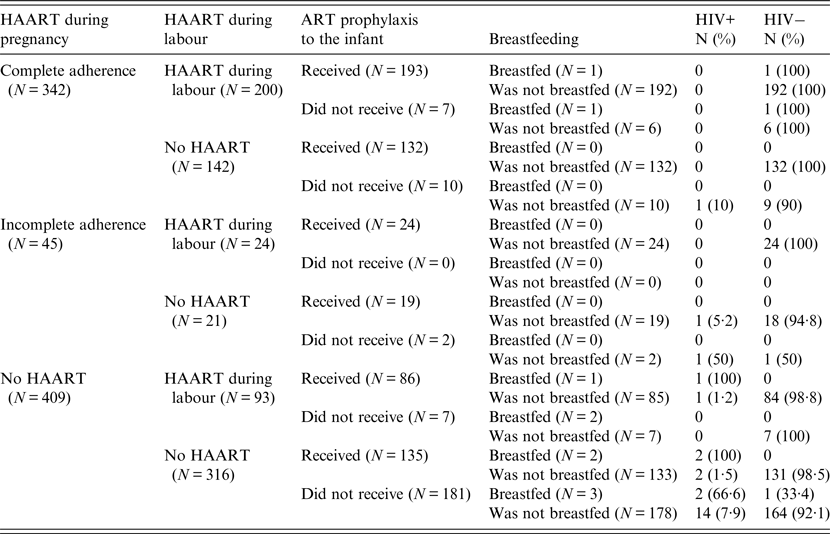
ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
Of all HIV-infected pregnant women in this study, 655 (82·3%) were of Ethiopian origin and their overall vertical transmission rate was 3·4% (Fig. 2). The rates of vertical transmission among the Ethiopian-born women have been reduced during the study period, while the rates of HAART among pregnant women and ART prophylaxis among infant have increased, P < 0·01. Complete HAART adherence among those who have been prescribed the regimen was 88·1%. Pregnant women of Ethiopian origin were also less likely to undergo caesarean section (N = 253, 43·8%) than other pregnant women born elsewhere (N = 74, 57·4%), P = 0·01.

Fig. 2. Number of infants born to HIV-infected mothers of Ethiopian origin in Israel, rates of vertical transmission, HAART for the mothers and ART to newborn, 1988–2011.
DISCUSSION
The overall vertical transmission rate in Israel during the 23 years of national follow-up was 3·1%, mostly occurring before HAART was introduced (16·5%) than after 1996 (1·7%). HAART during pregnancy decreased vertical HIV transmission significantly, and MTCT rates for complete adherence, incomplete adherence and no HAART were 0·3%, 4·5% and 5·3%, respectively, P < 0·01. Vertical transmission rates following the introduction of the HAART were low and consistent with reports from other developed settings [Reference Townsend9–Reference Forbes14].
With the substantial success of HAART in preventing MTCT, it is surprising that not all the pregnant women in our cohort took the therapy, especially since it is available in Israel for no charge. Incomplete adherence or no treatment resulted in higher MTCT rates, as failure to reduce vaginal viral load permitted in-utero or transvaginal transmission. It is possible that these women were prescribed the treatment but declined, or that it was not offered during the early years after the introduction of the HAART, when its benefit had not yet been established. A recent meta-analysis has found that the pooled proportion of pregnant women who adhere to the HAART was 62% (95% CI 50·1–73·3%) [Reference Nachega15]. This is a missed opportunity and has to be responded to with better adherence to the current MTCT prevention protocols by engaging HIV-infected pregnant women to HAART and medical care during the antepartum period. Adherence can be optimised by providing special attention to potential side effects and maternal concerns regarding possible negative pregnancy outcomes. Pregnant women should be informed that suboptimal application of HAART or poor uptake weakens its potency and may also lead to drug resistance.
The benefits of HAART in both preventing MTCT and reducing the progression of disease are well established, yet, the intervention should be weighed against concerns about drug toxicity and possible adverse pregnancy events. Previous publications have reported premature delivery and pre-eclampsia which was associated with prolonged use of antenatal of HAART [Reference Thorne and Newel4, Reference Townsend16, Reference Wimalasundera17].
Caesarean section has been found successful in reduction of MTCT, especially in cases when women received sub-optimal HAART or no therapy. This intervention should be evaluated with maternal viral load levels and the possible risks related to PROM. Additionally, HIV-infected women may be more likely to experience postnatal complications than uninfected women [Reference Read and Newell18]. In cases in which women received HAART, the rate of vertical transmission was lower for caesarean section deliveries, yet did not reach statistical significance in our cohort, as also found in Canada [Reference Forbes14].
The majority of the HIV-infected women in Israel were of Ethiopian origin. All pregnant women in Israel who are members of a known risk-group for HIV transmission in Israel, such as migrants from high HIV prevalence countries, are encouraged to be tested for HIV during the antenatal period [19]. If these women fail to demonstrate a negative HIV test result upon labour, a rapid test is usually performed in the delivery room at the hospital to allow the mother to receive ART or to recommend a caesarean section if necessary. HIV-infected mothers of Ethiopian origin are provided social support by community workers, as part of a planned governmental intervention, and formulas are provided for no charge to their newborns until 1 year of age. The reduction in vertical transmission rates during the study period in this community was apparent, as well as the increase in HAART uptake by pregnant women and increase in ART prophylaxis given to their newborns, which is probably a result of comprehensive intervention since 1996.
Although early HIV detection is recommended to allow early HAART and fertility treatment, late HIV diagnosis was not found in our study to have significant influence on vertical transmission. This might be due to the limited number of cases of vertical transmissions in our study or to the benefit of HAART even when prescribed late. HIV tests in Israel are provided free of charge in all medical settings, as well as in alternative non-medical community locations, both as a discrete or anonymous testing. The 87·5% of the HIV-infected women were aware of their HIV infection prior to pregnancy or labour. As most vertical transmission in Israel occurred among pregnant women of a known key risk-group, community interventions should be implemented in the community to encourage women who are members of these risk-groups to test for HIV early in each pregnancy [Reference Mor20].
PROM was previously associated with higher risk of MTCT during the pre-HAART era. Yet, this finding did not reach statistical significance in our study. A meta-analysis of 15 cohort studies suggested that a 2% increase in relative risk or transmission per hour of PROM, when the duration of PROM was <24 h [21]. However, HAART suppresses maternal viral load, thus decreasing the risk of MTCT, even during PROM [Reference Peters22].
Although it is the first study which includes all deliveries of HIV-infected pregnant women in Israel, it is subject to several limitations. First, maternal viral load was available only for 209 women. We therefore used CD4 levels in our analyses. Second, the exact products used for maternal HAART in the antepartum initiation were not available for all women, and we were not able to analyse the result by the specific drug provided. Third, women who were not diagnosed during pregnancy or immediately postpartum and whose infants remained unidentified were not included. However, as the study follow-up ended 4 years after the last child was born we had a long lead time period to allow late diagnoses.
In summary, as interventions for prevention of MTCT are widely available, the elimination of vertical transmission is a realistic public health goal. MTCT in Israel was mainly recorded among Ethiopian migrants, yet, in decreasing rates. Continuous efforts should be performed to encourage early HIV testing and allow effective HAART to pregnant women who are members of a key risk-group and ART prophylaxis to their newborns.
ACKNOWLEDGEMENTS
The authors wish to thank Ms Efrat Hadad and Ms Zehuvit Wiexelbom from the Department of Tuberculosis and AIDS in the Ministry of Health for their exceptional maintenance of the National HIV/AIDS Registry and accessing the medical files from the different hospital. The authors also thank Dr Illi Margalit for handling the data. This study did not receive any financial support.
DECLARATION OF INTEREST
None.









