Niger, a sub-Saharan country of 1 267 000 km2 with a population of about 14·8 million and centrally located in the African meningitis belt has regularly experienced meningococcal meningitis epidemics, mostly caused by serogroup A. The last major epidemic group A waves occurred in 1995 and affected 41 930 people of whom 3639 died [case-fatality rate (CFR) 8·7%], in 1996 with 16 745 notified cases (CFR 9·4%), and in 2000 with 14 633 cases (CFR 7·5%). Since then, serogroup A has remained predominant although without causing large epidemics in Niger. In the first weeks of 2009, a notable increase in clinical cases was reported to the national surveillance system (DSSRE) (Fig. 1). By week 5 the suspected cases reached 1024, with 65 deaths (CFR 6·3%) registered by the DSSRE. At that time, the health district of Gaya (southwestern part of Niger) crossed the epidemic threshold and three health districts in the Tahoua (inner part of the country) and Zinder (southeastern, on the border with Nigeria) regions crossed the alert threshold. Microbiology of CSF performed at the Centre de Recherche Medicale et Sanitaire (CERMES), which is in charge of the national microbiological surveillance, showed that Neisseria meningitidis serogroup A was the predominant causative agent (see below). Outbreaks progressed to other parts of the country; the epidemic peak was observed from the 12th to the 15th week with respectively 1338, 1364, 1432 and 1521 clinical cases. Regional attack rates varied between 8·5 and 9·3/100 000 inhabitants but attack rates higher than 30 were observed at district level (Madarounfa, weeks 12–14; Boboye, week 15). By week 15, most health districts still affected by the epidemic were located in southern Niger. By week 19, the last health districts crossing the epidemic threshold were located in the extreme western (Tillabery) and eastern (Abalak) parts of the country.
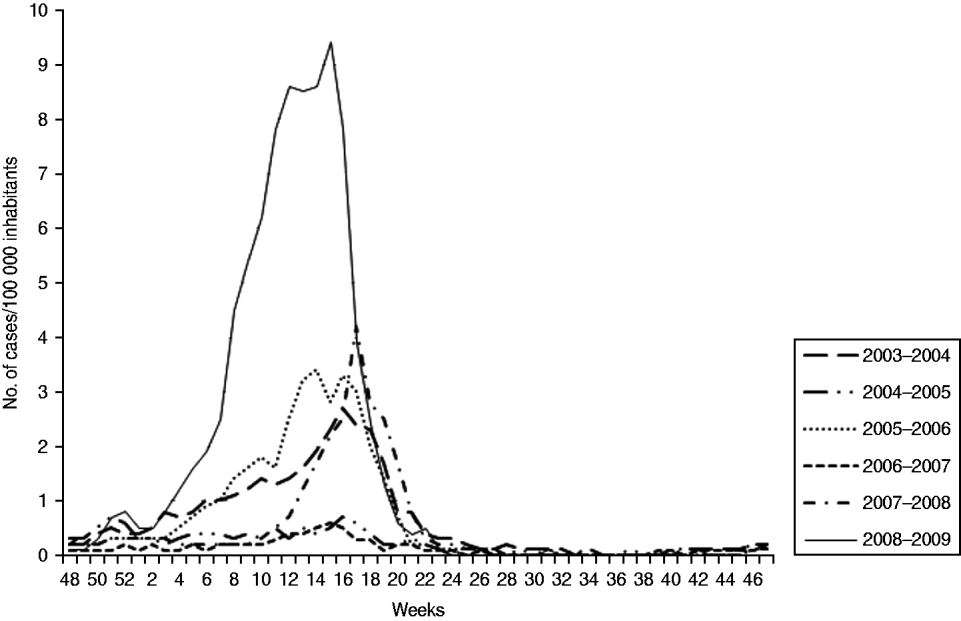
Fig. 1. Attack rates of meningitis in Nigeria over the years 2003–2009. (Data collected by the Direction of Statistics, Surveillance and Response to Epidemics – Ministry of Public Health.)
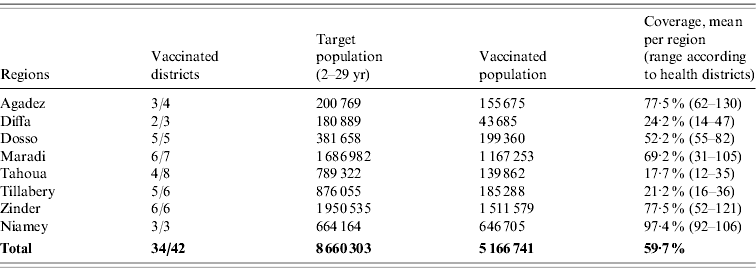
In response to the serogroup A meningococcal epidemic, surveillance was rapidly strengthened and case management using standard antibiotics (oily chloramphenicol, ceftriaxone or ampicillin when available) was facilitated by free treatment of meningitis cases and pre-positioning of care materials, antibiotics and antipyretics at regional, district and healthcare facility level. Based on the serogroup determination and epidemiological surveillance, reactive mass vaccination campaigns were launched by the Ministry of Public Health (MoPH) with the support of WHO and partners, including Médecins sans Frontières and UNICEF. These were targeted not only at districts that had reached the epidemic threshold, but also in those in alert status and adjacent to an epidemic district. The population of the capital (Niamey Region) unvaccinated since 2001 was also vaccinated although these districts had not reached the alert threshold. The target population for immunization was aged 2–29 years. The stockpile at the beginning of 2009 was 822 650 doses of bivalent (A+C) vaccine and complementary doses were provided by the International Coordinating Group (ICG) on Vaccine Provision for Epidemic Meningitis Control. Mass vaccinations allowed for progressive control of the outbreaks in most parts of the country. Finally 5 166 741 doses were administered to the whole country with vaccination coverage varying between 17% and 97% of the target population according to region (Table 1).
Table 1. Vaccine response to the N. meningitidis serogroup A 2009 epidemic
By week 26, when the incidence of meningitis had fallen to pre-epidemic levels, a total of 25 out of 42 health districts in Niger had reached the epidemic threshold and 11 the alert threshold. Modelling of the spatial-temporal evolution of the epidemic at health-centre level failed to show a definite progression in the dynamics of meningitis-confirmed cases (data not shown). Finally from 1 January to 28 June 2009 a total of 13 357 clinical cases of meningitis were reported with a CFR of 4·2% for the country, the highest CFR (11·4%) being observed in the Niamey Region and the lowest in Zinder Region (2·3%). The high CFR in Niamey could be explained by several factors, among which were a more accurate diagnosis in the hospitals of the capital with fewer patients who did not have meningitis being included in the incidence statistics, and admission to the hospitals of those at the very last stages of the disease due the hesitation of the family in view of the related costs (transport and healthcare, even if these latter cases are free during a meningitis epidemic, and for children aged <5 years).
During the same period, CERMES which runs the microbiological surveillance for the country received 3765 CSF specimens representing 28·1% of the notified cases whereas between 2005 and 2008 CSF testing represented 70–100% of the notified cases. Reasons to explain the reduction in collecting CSF samples during a large epidemic include: lumbar puncture needles and collecting tubes being out of stock; overburdened healthcare structures; being taken into care and treatment on the basis of clinical diagnosis; and over-notification of cases.
After culture of fresh specimens or trans-isolates (n=123) and PCR (all samples) targeting the main causes of bacterial meningitis (N. meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae) [Reference Chanteau1], 51·5% (n=1939) of CSF specimens remained negative. Among positive CSF specimens, N. meningitidis was by far the most predominant (n=1683), followed by S. pneumoniae with 73 cases; H. influenzae accounted for only 11 cases, of which seven were of type b.
Of 1683 confirmed meningococcal aetiologies, the most frequent was serogroup A (n=1641, 97·5%). Serogroups W135 (n=10) and X (n=15) accounted respectively for 0·6% and 0·9% of the meningococcal cases; a single case attributed to serogroup Y was detected by PCR, and the serogroup could not be identified for 16 specimens (0·95%).
All cultured meningococcal strains (n=54) were analysed for their antibiotic susceptibility according to the recommendations from the Antibiogram Committee of the French Society for Microbiology (CA-SFM). They were susceptible to all tested antibiotics but especially to β-lactams (penicillin, amoxicillin, ceftriaxone) and chloramphenicol, supporting the appropriateness of WHO recommendations for antibiotic treatment [2].
A set (n=26) of serogroup A meningococcal strains collected during four distinct field investigations in districts with epidemic (Gaya, Dosso, Maradi and Zinder which represent very distant places) or collected in the National Hospital of Niamey was selected and sent for multi-locus sequence typing (MLST) to the WHO Collaborating Center in Marseille, France. MLST indicated that all but one belonged to sequence type (ST)7. The sole strain belonging to ST2859 was isolated in Niamey, whereas ST7 strains came from the health districts of Dosso, Niamey, Maradi and Zinder. The CSF specimen positive for serogroup Y was also analysed and typed as ST8391 from clonal complex (CC)167.
In Niger, while the 1995 and 1996 major epidemics were due to serogroup A ST5, they were followed in 1999 and 2001 by outbreaks involving a mixed population of ST5 and ST7 strains, both sequence types belonging to ST5 CC5 of subgroup III. ST7, which differs from ST5 at one locus, had also been identified in Cameroon, Chad and Nigeria and has gradually replaced the ST5 initial clone in all the countries of the ‘meningitis belt’ [Reference Nicolas3]. By 2002, serogroup A ST7 strains had totally replaced the ST5 clone [Reference Caughant and Nicolas4, Reference Nicolas5]. In 2003, a new variant of ST5 CC5 of subgroup III, ST2859, emerged in Burkina Faso and expanded gradually, causing epidemics in 2006 and 2007 involving more than 19 000 and 30 000 clinical cases, respectively [Reference Teyssou and Muros-Le Rouzic6]. In Niger in 2007, sequence typing performed on 17 serogroup A strains showed 11 ST7 and six ST2859. Up to 2007 no isolate of ST2859 meningococcus had been reported in Niger, and interestingly, all of the ST2859 strains were detected in the southwest of Niger bordering Burkina Faso (five strains from Tillabery Region and one from the capital Niamey); whereas all of the ST7 strains were isolated in the southeast of the country, with the exception of one strain isolated in Niamey [Reference Nicolas7]. In 2009, most meningococcal strains analysed by MLST belonged to ST7 indicating that ST7 strains remained predominant in Niger whereas they were replaced by ST2859 in Burkina Faso. This close microbiological surveillance is therefore crucial to better understand the epidemiology of meningococcal meningitis in Africa and to monitor the spread and evolution of the more virulent sequence types.
Concomitantly with the epidemic in Niger in 2009, Nigeria, which shares its northern border with Niger, also experienced an unprecedented epidemic which started in the northwestern part of the country and spread eastwards to the northeastern part of the country [8]. At week 25 (21 June), 55 739 suspected cases of meningococcal disease, including 2448 deaths (CFR 4·4%) were reported by the MoPH [9]. Nevertheless, N. meningitidis serogroup A was less prominent than in Niger (88%). CSF specimens positive for N. meningitidis serogroup W135 were also found in 12% of the reported cases. In Chad, another neighbouring country (eastern border), the MoPH reported from 29 December 2008 to 21 June 2009 1329 suspected cases of meningococcal disease including 142 deaths (CFR 10·7%) [9]. Of 288 collected CSF specimens, 58 were positive for N. meningitidis serogroup W135 and 45 specimens positive for N. meningitidis serogroup A, suggesting that both serogroups were responsible for the epidemic in Chad. On the other hand, Burkina Faso, sharing its eastern border with Niger, declared up to week 25, 4013 suspected cases of meningococcal disease, including 514 deaths (CFR 12·8%) [9]. The laboratory results on 275 CSF specimens indicated 78 S. pneumoniae, 36 N. meningitidis serogroup A and three N. meningitidis serogroup W135 – the remaining specimens tested negative.
Reasons for the higher CFR in Chad remained unclear but the presence (56%) of N. meningitidis serogroup W135 [8] could be one of several factors. In Burkina Faso, the higher CFR is probably due to the presence of S. pneumoniae as the main aetiological agent, which is known to cause a much higher mortality [Reference Yaro10]. The biological results from these three neighbouring countries showed proportions of aetiological agents different from Niger, where the main agent identified in CSF was N. meningitidis serogroup A (97·5% of meningococcal meningitis). Very few N. meningitidis of serogroups X and W135 were found during the course of the epidemic and S. pneumoniae was identified in only 4% of positive CSF specimens. Finally, Nigeria and Niger were the most affected countries in West Africa during the 2009 season, together accounting for more than 85% of the cases [8, 9].
These epidemics occurred just before the introduction of a new group A conjugate vaccine (Meningitis Vaccine Project) in the countries of the African meningitis belt. This new vaccine (MenAfriVac) has been shown to be more efficacious than the current polysaccharide vaccines during clinical trials, particularly in infants [Reference LaForce11]. It induces protection of longer duration and (hopefully) is thought to reduce nasopharyngeal carriage as experienced by the introduction of monovalent MenC conjugate vaccine in the UK [Reference Campbell12]. Mass vaccination campaigns with this vaccine started in September 2010 in Niger, as well as in Burkina Faso and Mali and will take place in four consecutive waves until November 2011. However, due to the relatively high vaccine coverage obtained in five of the eight regions in 2009 (except in the Diffa, Dosso and Tahoua regions, Table 1) and speculating on the spacing between major waves of meningitis epidemics in Africa, one can hope that the 2009 epidemic will be the last epidemic wave of N. meningitidis serogroup A in Niger before the introduction of the new conjugate vaccine.
ACKNOWLEDGEMENTS
This work was financially supported by French Ministry of Foreign Affairs (FSP no. 2005-174) and by Sanofi Pasteur (contract Men07).
The authors are indebted to all the doctors and health staff who have sent CSF specimens and epidemiological forms to CERMES and also to the DSSRE staff. We thank Bassira Issaka, Lagaré Adamou, Amadou Moussa, Djibir Zanguina, Sani Ousmane, Issaka Seyni, Moumouni Ousseini and Adama Moussa for technical support, Noémie Phulpin, Ramatou Yahaya, Ali Sidiki and Florian Girond for database management and Amina Amadou and Ali Elhaj Mahamane for RDT production, distribution and training. We also thank the staff of WHOCC IMTSSA (Marseille, France) for MLST typing of meningococci strains. Thanks are also due to Professor Musa Hassan King for reading and commenting the manuscript.
DECLARATION OF INTEREST
None.




