INTRODUCTION
Helicobacter pylori is a common bacterial pathogen infecting half of the human population worldwide. Its prevalence varies according to the stage of a country's development, ethnic background and various socioeconomic factors [Reference Bruce and Maaroos1–Reference Brown4]. The population of Brussels is cosmopolitan with a variety of ethnic backgrounds and different levels of socioeconomic status [Reference Rea, Delwit, Rea and Swyngedouw5]. To date, only few studies have assessed H. pylori infection prevalence in Belgian populations, particularly in adults [Reference Blecker6, Reference Blecker7]. We conducted this large retrospective, observational study in order to analyse the epidemiology of H. pylori infection in the Belgian population during the last 20 years. The study included children and adults attending several digestive endoscopy centres in Brussels. Isolation of the bacteria by culture was the only diagnostic criterion for the presence of a H. pylori infection.
In the analysis of the variability of infected patients in different populations, we took into account the time-course effects of transition epidemiology linked to the immigration of populations with different ethnic backgrounds and the relationships of the movement of these populations with the well-known effect of the decrease of H. pylori prevalence in developed countries.
METHODS
Patients and setting
The source of data collection was the medical laboratory of the Brugmann University Hospital in Brussels. Since 1984 the hospital has served as the reference centre for culturing of H. pylori for a large number of centres including public university hospitals (Brugmann University Hospital, Queen Fabiola Children's University Hospital, Saint-Pierre University Hospital, Institut Bordet Cancer Centre), public hospitals (Hôpitaux Iris Sud) and private hospitals (CHIREC/Clinique de la Basilique & Edith Cavell, Cliniques de l'Europe, Clinique du Parc Leopold) as well as for outpatient clinics and private gastroenterologists. These hospitals represent a large proportion of the hospital beds in Brussels (3147/9175 beds) [Reference Taymans8] and also perform routine gastrointestinal endoscopies on an outpatient basis. Patients originated from a number of Belgian cities, with the majority from Brussels (80%). Available demographic (including ethnic origin) and clinical data were collected for each patient. The indication for gastrointestinal endoscopy included various gastrointestinal complaints. Only patients with at least one interpretable result (either positive or negative) of H. pylori culture were included.
Specimens
The study included gastric biopsies (from antrum or antrum and corpus) according to the referring centre from every request for H. pylori culture. Concomitant biopsies in the same patient were considered as one set of biopsies leading to a single culture result (positive result=at least one biopsy of the set being positive). Samples treated from 1 January 1988 to 31 December 2007 were included. On arrival at the laboratory, the biopsies were immediately processed or frozen at −70°C until required.
H. pylori isolation
Immediately before culturing, each biopsy specimen was ground in sterile distilled water. The final suspension was inoculated by circular streaking with a bent pipette on in-house selective agar plates. During the first year of the study, a serum-based medium containing activated charcoal was used for isolation of H. pylori [Reference Glupczynski, Labbe, Thiabaumont, Megraud and Lamouliatte9]. However, since 1989 a single selective home-made medium has been used. This medium consists of 10% horse blood medium supplemented with several antimicrobial agents. A triphenyltetrazolium chloride was also added for easier detection of H. pylori, based on the golden appearance of the colonies [Reference Queiroz, Mendes and Rocha10]. Plates were incubated for 3–7 days at 37°C under a humid, microaerobic atmosphere. The culturing was extended to 10 days if there was a known positive urea test. The typical growth of H. pylori was checked after 3 days, then on a daily basis thereafter. The result of the culture was recorded as positive, negative or uninterpretable (technical incident or overgrowth). Uninterpretable results (n=90) were discarded from the analysis. The number of colonies per millilitre was also recorded. Compared to urea breath test and histology, this in-house culture method yielded 98% sensitivity and 100% specificity [Reference Cadranel11].
Data analysis
All data relating to patients (demography, H. pylori culture, clinical status) were retrospectively transferred into Excel 2000 software. The databank was analysed with Epi Info (Centers for Disease Control and Prevention, USA). Only the first culture result from the first upper gastrointestinal endoscopy of each patient was included in order to accurately determine the rate of H. pylori infection.
Age
Median values are given, as age (range 1–99 years) was not normally distributed.
Ethnic background
Ethnicity was determined by available demographic data [clinical information, medical report, endoscopy unit's fiches, Medical viewer®, Mediweb® (Agfa-Gevaert Group, Belgium)]. If ethnicity could not be determined, the patient was included in a separate ‘undetermined group’ including unknown, mixed, and rare ethno-geographical backgrounds. Results from Caucasian Western European patients were compared to those of foreign ethnicity, particularly the North African group which was the most numerous group of patients.
Time trends
Marching cohort effect was determined by comparing the secular trend in the proportion of infected patients in subjects grouped according to their birthdates.
Three effects might have had an effect over time: changes in age distribution, differences in ethnic distribution, and some cohort effects.
Dynamic of infection
As H. pylori infection is a chronic disease, without clear evidence of immune control or spontaneous recovery in the absence of specific therapy, a SI model was expected to better fit the data (S=Susceptible; I=Infected/Infectious), without a third R (Recovered) compartment.
According to this model, the number of infected subjects in a closed population increases as a logistic function. Due to the transversal design of the study, it was not possible to determine the incidence of H. pylori in the population. This study is therefore based on the microbiological analysis of biopsies referred to our laboratory by several clinicians. Although the clinicians may have changed their clinical criteria, it can be assumed that as they operated in the same referral centres, the indications for endoscopies remained stable during the study period, i.e. mainly heartburn, pain, vomiting, and bleeding or anaemia.
We were unable to estimate whether the symptomatic cases presenting at the clinics reflect the total of symptomatic cases, or whether there is a ‘healthy’ selection bias – in the latter case, true incidence of H. pylori infection would be greater than the incidence observed.
Even if the selection criteria for gastrointestinal endoscopy and prescription of H. pylori culture differ greatly with age (Fig. 1), it can be assumed that the proportion of positive cultures in gastric biopsies is representative of the proportion of positive cultures within the entire population, whether biopsied or not.
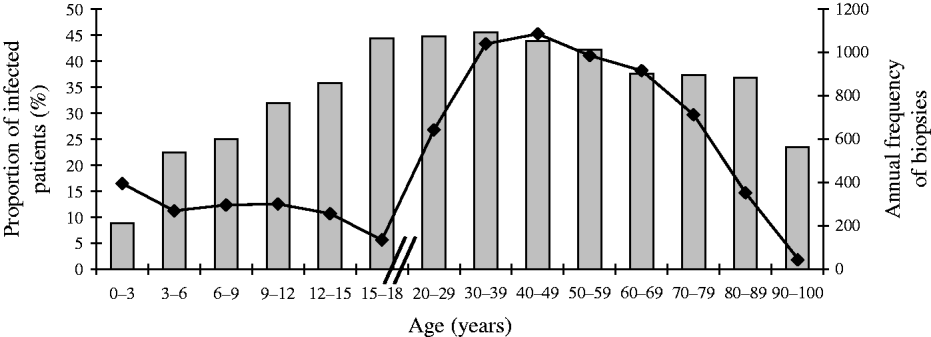
Fig. 1. Progression with age of the proportion (%,![]() ) of first gastric biopsies with positive culture for H. pylori and annual frequency (–◆–) of first biopsies with culture for H. pylori in Brussels as a function of age. Total number of first biopsies=22 612.
) of first gastric biopsies with positive culture for H. pylori and annual frequency (–◆–) of first biopsies with culture for H. pylori in Brussels as a function of age. Total number of first biopsies=22 612.
With this assumption in mind, the ratio of the proportion of H. pylori-infected patients between two age groups is a correct estimate of their ratio of incidence. Without knowing the true incidence, it is possible to sketch the dynamic of infection with age using this approach.
RESULTS
Table 1 shows the distribution of the 22 612 persons divided in two age groups and grouped according to ethnic origin. In the younger age group (⩽18 years), the Western European and the Italian groups were infected at a proportion of 8·4% and 12·3%, respectively, at markedly lower percentages than in other ethnic groups (range 23·3–46·8%). In adults, one third (31·5%) of the Western European group was infected, also at a markedly lower percentage than in the other ethnic groups (range 41·9–68·5%).
Table 1. Distribution of patients with median age according to geographic origin
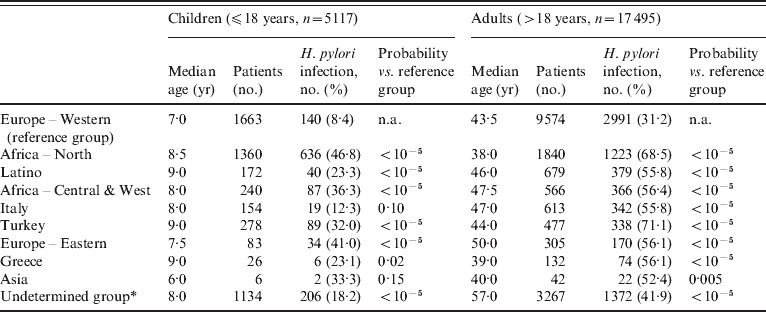
n.a., Not applicable.
Percentages of patients infected with H. pylori grouped according to age and geographic origin. Probability refers to the statistical significance of comparison with Europe – Western as the reference group (χ2 test with Yates' correction).
* The Undetermined group includes patients with unknown, mixed, and rare ethno-geographical backgrounds.
Figure 1 shows within each age group both the annual number of first biopsies with H. pylori culture according to age and the proportion of positive cultures. The annual number of gastric biopsies (Fig. 1, diamond symbols) decreased across the childhood groups: 393 in the infant group (<3 years), 300 in the other children age groups, and 135 in the older adolescent group (15–18 years). Then, the number of biopsies by age sharply increased reaching 700 in young adults (20–29 years) and about 1000 in other age groups. In the elderly (⩾70 years), it fell by about 300 according to decade of age.
However, the proportion of H. pylori-positive cultures (Fig. 1, grey bars) increased regularly with age. It ranged from 9% (104/1179 patients) in the youngest age group (0–3 years) to 45% (180/406 patients) in the adolescent group (15–18 years), remained quite stable at 45% (1410/3116 patients) in the 30–39 years group, then decreased gradually to 38% in the 60–69 years group, and fell markedly to 23% (29/124 patients) in the oldest age group (>90 years) (P<10−5 for trend).
Gender and H. pylori infection
The proportion of H. pylori-infected patients was virtually the same in males (37·1%) and females (36·1%). The difference was only statistically significant in adult patients aged 20–40 years (50% vs. 40%, P<10−5).
Marching cohort
In 1988, at the beginning of the study, the proportion of infected patients in the total population was 43% (375 positive out of 865 patients); 20 years later, it had fallen to 29% (428 positive out of 1477 patients) (P<10−5). We analysed whether changes in age distribution, modifications linked with ethnic background, and cohort effects could have been factors in this marked decline over time.
Figure 2 shows the changes over time in the proportion of infected patients according to ethnic origin. A decrease was observed in all ethnic groups (P<10−5). However, all age subgroups showed a decreased prevalence of H. pylori infection between 1988 and 2007 (data not shown), except for the youngest subgroup (0–9 years). This paradox was due to a significant heterogeneity observed within this age group when stratifying it according to ethnic origin (Fig. 3). Indeed, in Western European children, the proportion infected was very low (<10%) and gradually decreased over time. In North African children, on the contrary, it started at 31% during the years 1988–1991; increased to 41% during 2000–2003 and then decreased slightly thereafter to 34%.
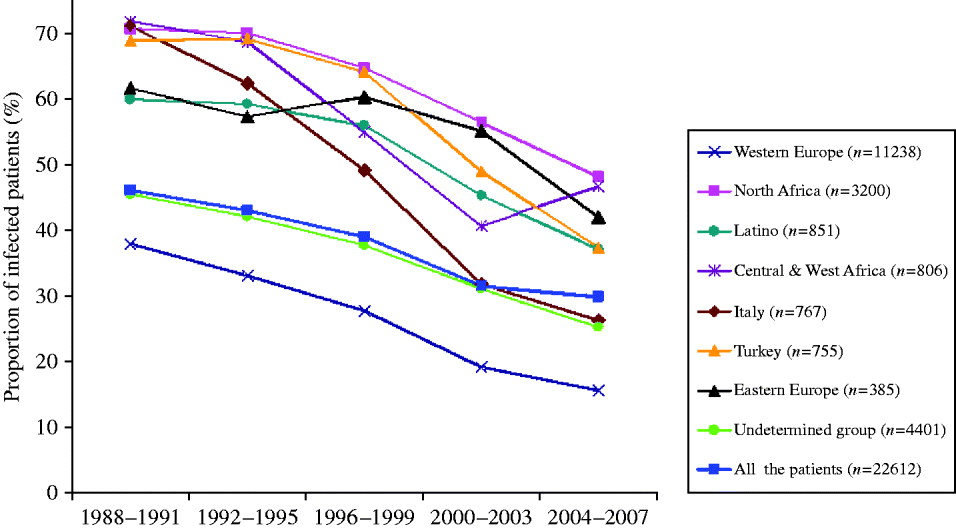
Fig. 2. Progression with time from 1988 to 2007 of the proportion of first gastric biopsies with positive culture for H. pylori according to geographic origin. Data of the less numerous groups of patients (Greece and Asia) are not shown. The decrease (2004–2007 vs. 1988–1991) was significant (P<10−5) within each geographic group. Total number of patients (n=22 612) is represented in the overall curve appearing with blue squares.
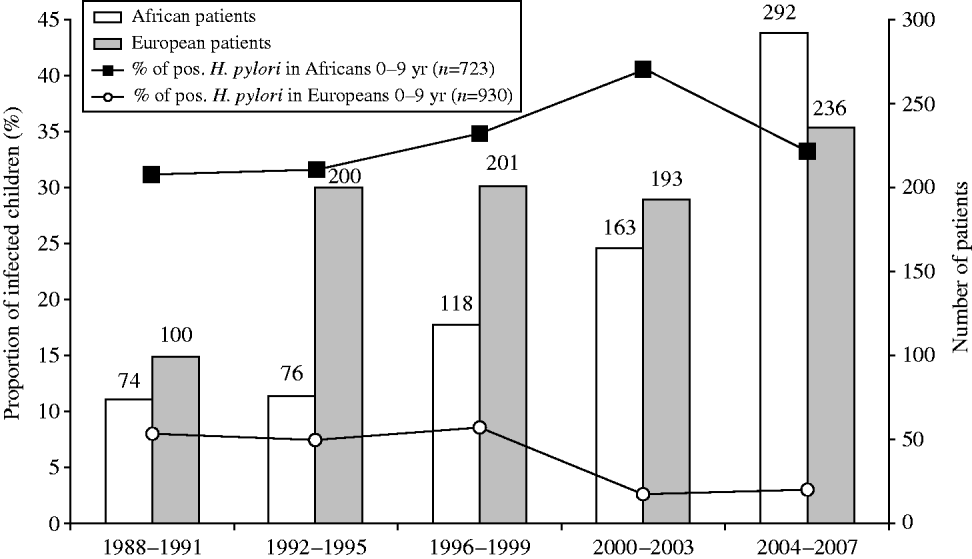
Fig. 3. Progression with time from 1988 to 2007 of the proportion of first gastric biopsies with positive culture for H. pylori in Western European (
![]() ) and North African (□) groups of children aged 0–9 years (n=1653 patients). Number of positive H. pylori cases in African (–▪–) and European (–○–) children.
) and North African (□) groups of children aged 0–9 years (n=1653 patients). Number of positive H. pylori cases in African (–▪–) and European (–○–) children.
The pattern of progression of infection with age (Fig. 4) differed markedly between the two groups: in the North African group the rate of increase was more pronounced during childhood and led to a high infection prevalence (72%) in adults (⩾18 years), whereas in the Western European group, the progression was continuous and at a low pace in childhood, adolescence, and adulthood. As the proportion of positive H. pylori cultures in adulthood is closely related to the cumulative incidence (Y max) in a SI model, we calculated from this cumulative incidence, an estimated hazard rate (eHR) for the two groups using the formula: y=Y max*[1−exp (−eHR*t)]. The formula for North African subjects was
and for Western European subjects
While the accuracy of these eHRs (0·15/year and 0·04/year) is impossible to determine, it is plausible that their ratio – the relative risk 3·75 – is a good estimation of the increased risk of H. pylori infection in subjects of North African origin vs. subjects of Western European origin.
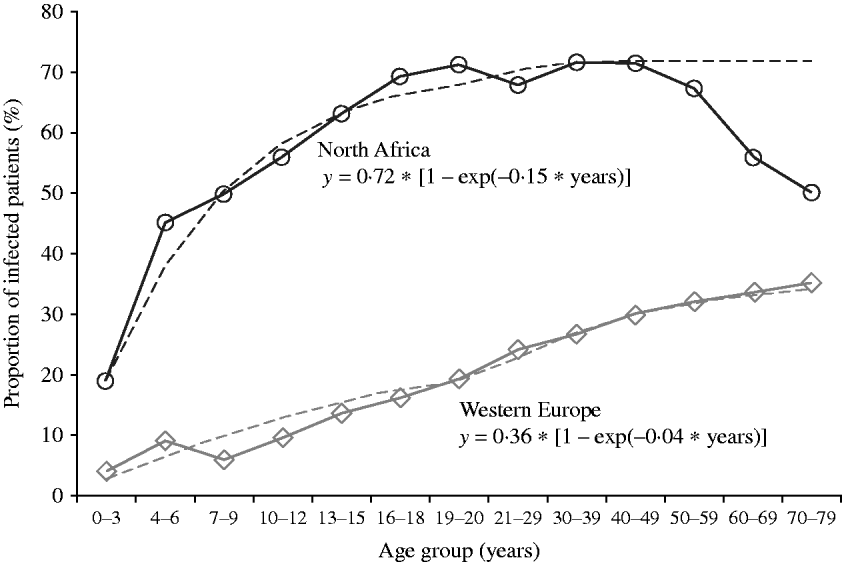
Fig. 4. Observed data of the progression with age of the proportion of first biopsies with culture positive for H. pylori in subjects living in Brussels of Western European (n=11 238) or of North African (n=3200) origin. The variation with age was fitted to a saturation function: y=Y max*(1−exp (−eHR*t)), where Y max is the maximal proportion of first biopsies positive for H. pylori in both groups, eHR is the estimated hazard rate, and t is the age in years.
DISCUSSION
Acquisition of H. pylori infection during lifetime
The aim of this study was to determine the dynamic of H. pylori infection in a large population attending gastroenterology clinics around Brussels over the last 20 years according to several parameters such as secular trend, age, gender, and major ethnic group. This historical observation confirmed that the number of H. pylori-infected subjects increases until the age of 18 years in a population with high prevalence [Reference Bruce and Maaroos1]. However, in a population with low prevalence, the cumulative proportion of infection increases linearly throughout life, possibly as a result of (generation) cohort effect combined with constant risk (hazard rate). The selection of subjects was based on spontaneous referral to an outpatient clinic for abdominal symptoms and performance of an upper gastrointestinal endoscopy with biopsies for H. pylori culture. According to this mode of recruitment, it is not possible to give an estimate of the prevalence or the incidence of H. pylori infection in Brussels. By assuming that the symptoms justifying referral to an outpatient clinic are homogeneous over the lifetime period (at least until age 60 years; since the decreasing proportion of H. pylori-positive biopsies after 60 years is probably due to competitive risk of other digestive diseases), and considering the relatively stable total number of patients in this age range, it is possible to estimate the ratio of incidence of H. pylori infection from early childhood to late adulthood (60 years) in North African subjects on the one hand, and in Western European subjects on the other – the two predominant groups with, respectively, the greatest and the lowest proportion of H. pylori infection (Table 1). With the assumptions of similar selection criteria in the two groups, the incidence of H. pylori infection in North African children is 3·75 times greater than that in Western European children (Fig. 4). Our observational data strongly confirm that in immigrants H. pylori infection is acquired during childhood and adolescence, and that adult patients – even when consulting at an older age for the first time – have acquired their infection well before adulthood. Indeed endoscopies are far more frequent in adults than in children and adolescents (Fig. 1). Therefore it is not possible, from our data, to distinguish whether the difference is due to different symptoms or different clinical interventions. In native Brussels subjects, the risk of acquiring H. pylori infection seems stable over most of the lifetime (Fig. 4). These aspects of the dynamics of H. pylori infection possibly explain that therapeutic interventions aimed at eradicating this pathogen in adults are not clearly associated with a public health benefit in a reduction of the incidence of stomach cancer [Reference Herrera and Parsonnet12]: a strategy of intervention during childhood remains to be tested and might prove more efficient in preventing stomach cancer.
Secular trend of decrease of H. pylori infection
Evidence is emerging in different populations, including in developing countries, that H. pylori prevalence is declining in all age groups [Reference Tkachenko13, Reference Daugule and Rowland14]. In this study, the trend of decrease was almost the same for the different ethnic groups (Fig. 2). This is probably related to an improvement in the standard of living and an increased use of H. pylori eradication therapy in the global population. A significant decrease in different ethnic groups was observed from 1988 to 2007: Western European subjects expressed a significantly lower infection rate during the whole period of observation, declining from 36·1% (173/479 patients) in 1988 to 14·1% (67/475 patients) 20 years later, compared to the corresponding rates in North African subjects of 71·5% in 1988 and 49% in 2007 (P<10−5). The data obtained in the immigrant populations are comparable to those of their compatriots living in their country of origin during the same period [Reference Brown4, Reference Mandeville15–Reference Banatvala23]. Surprisingly, the most characteristic feature of our study was that the proportion of patients infected with H. pylori is not declining in all age groups. Indeed, the age-related epidemiology of H. pylori in our population did not show a decrease in prevalence over the years in children aged ⩽9 years. A comparison of the age-related prevalence for the same children with an additional criterion (ethnic background) provides an explanation. This particular evolution is probably related to a very high proportion of infected North African children (>30%) amplified by an increase in the number of these children over the study period (Fig. 3). This tendency is reinforced since we observed that compared to Caucasian Europeans, patients originating from developing countries were younger and exhibited higher infection rates (Table 1) and is probably related to socioeconomic differences as described in a study on H. pylori infection in children in Texas [Reference Opekun24]. Indeed, research activity based on the ‘mind mapping’ of young people in Brussels demonstrates the separate social networks of young people who do not mix with each other [25].
We observed a lower proportion of infected females compared to males, which was highly significant in adults. These findings are in agreement with a similar Korean study [Reference Kim26].
The extrapolation of results obtained in a symptomatic population to the general symptom-free population is obviously controversial. A recent study in Latvia shows that adjusting for age, the prevalence of H. pylori infection was not higher in symptomatic children compared to asymptomatic children of the same age [Reference Daugule27]. On the other hand, a lower prevalence of H. pylori infection has been described in patients with dyspeptic symptoms compared to non-symptomatic individuals [Reference Shi28].
As we had no precise denominator for the coverage of the population from our laboratory data, we were unable to extrapolate the prevalence of H. pylori infection in the global population of Brussels.
To our knowledge, this is the first report of an epidemiological study including both adults and children where H. pylori infection is defined only by a positive culture which is considered to be the most specific method [Reference Megraud29]. Despite the availability of non-invasive tests in recent years, the conduct of the main centres involved in this study remained the same regarding the use of H. pylori culture. Therefore, this method can not be viewed as an important source of classification bias due to technical change over the years of the study. On the other hand, its routine use offers the advantage of allowing antibiotic susceptibility testing, strain typing and further studies which combine the prevalence of the infection with other topics that require isolation of the bacterium.
Since the indications for upper gastrointestinal tract endoscopy are different between adults and children, we can not exclude that children and adults were examined for H. pylori with the same clinical criteria. If there is clinical misclassification between adults and children, we believe that the proportion of positive H. pylori biopsies in children would still be greater, which gives credence to our findings.
CONCLUSIONS
During this observational study spanning twenty consecutive years in more than 22 000 subjects:
(1) The proportion of H. pylori-infected adult patients attending endoscopy clinics in Brussels decreased significantly, confirming the declining prevalence of H. pylori infection in developed countries.
(2) The period of maximal risk of acquiring H. pylori infection in the group of North African origin extends over childhood and adolescence, and the cumulative proportion of infected subjects reaches a maximum at around 18 years, with no further increase in adulthood – suggesting that most subjects have already acquired their infection before adulthood. This could be of major public health relevance (see below). By contrast, in the Western European group, the cumulative proportion of infection increases steadily over the whole lifespan, at a markedly lower level, suggesting a more or less constant and stable rate of infection throughout life.
(3) This study highlights the important variability of H. pylori infection status even in persons living in the same country. Compared to Caucasian Europeans, patients of African ethnicity showed a significantly higher proportion of H. pylori infection, particularly young children where the infection rate has remained high over the latter years.
(4) Further studies are required to assess H. pylori prevalence in the general population.
ACKNOWLEDGEMENTS
We thank the gastroenterologists and the members of the laboratories who contributed to the collection of data. Particular thanks are due to Professor Y. Glupczynski for critical review of study proposals and Dr B. Boland for careful revision of the manuscript. This work would not have been possible without the Epidemiology of Infectious Disease course organized annually at the Imperial College, London, and attended by one of us in 2005.
DECLARATION OF INTEREST
None.







