INTRODUCTION
Diarrhoea causes an estimated 2·5 millions of deaths per year and remains a major cause of illness among infants and young children worldwide [Reference Kosek, Bern and Guerrant1]. Rotavirus is estimated to cause between 1·0 and 4·8 hospitalizations/1000 children aged <5 years in Spain [Reference Gil2–Reference Luquero Alcalde4]. Information on the epidemiology of agents causing diarrhoea is necessary to establish adapted control measures (e.g. Rotavirus vaccination).
Aragon is an autonomous region in the north of Spain with 1·3 million inhabitants (National Institute of Statistics) [5]. With around 120 000 annual cases diarrhoeal illness is the most frequently notified disease (98/1000 inhabitants per year). In 2006 three surveillance systems for diarrhoeal illnesses were in place in Aragon: syndromic, laboratory and agent-specific surveillance.
The syndromic surveillance system is a mandatory system in which cases of diarrhoea with presumed infectious origin are notified on a weekly basis by all physicians in the Aragon region. The number of notifying physicians, the data flow and case definitions of the syndromic notification system have remained stable since 1998. The syndromic surveillance system commenced in 1982 with the objective of describing trends of diarrhoeal diseases and detecting outbreaks in order to implement control measures. This system has never been evaluated.
The laboratory surveillance in Aragon is based on two laboratories from university hospitals in Zaragoza (capital of Aragon). Since 1989, on a weekly basis, these two laboratories notify all positive tests to the Spanish National Centre for Epidemiology. The laboratory surveillance system covers 40% of the population of Aragon. Laboratory surveillance is part of the Spanish surveillance network that aims to follow trends at the national level. Consequently the laboratory data are currently analysed at national level and not used at the regional level.
For the agent-specific surveillance system physicians notify the total number of cases of Vibrio cholera, bacillary dysentery and bacterial foodborne diseases on a weekly basis. From 2001 to 2006 two cases of cholera and 11 cases of bacillary dysentery were notified. The number of bacterial foodborne disease notifications did not increase.
In the period 1982–2004 the syndromic surveillance system did not detect any alert and did not trigger any public health action. In 2005 and 2006 epidemiologists of the government of Aragon noticed an increase of 10% in the yearly number of diarrhoea cases compared to previous years (average 114 000 cases between 1998 and 2004). Moreover, a change in the seasonal pattern with an increase in the winter months was observed. The data from the syndromic surveillance system were insufficient to identify the causative agent of this increase or the affected groups and no direct public health action could be taken.
We conducted a study to describe the increase in notified diarrhoeal illness cases, and to identify the organisms responsible for this increase in 2005 and 2006 in order to give recommendations to guide control measures and to strengthen surveillance. Our hypothesis was that a microorganism that was regularly tested in the laboratories of Aragon was responsible for the recent increase. We selected the period 1998–2004 as the reference as in this period there were no alerts from the syndromic surveillance system. In addition the laboratories would still be able to recall changes in detection methods for 1998–2004. Due to the low number of cases of cholera and bacillary dysentery and the stability in the notification of bacterial foodborne cases, we focused our study on the syndromic and laboratory surveillance system and did not consider the agent-specific surveillance system.
METHODS
Syndromic surveillance system
We described the increase of diarrhoea notifications using the cases reported to the syndromic surveillance system between 1998 and 2006. We assessed the quality of the data by identifying missing data and potential errors like outliers (e.g. single weeks with high values). We described the total number of cases and the mean and range of the number of weekly notified cases over the years. To identify changes in seasonal patterns, we computed a 13-week moving average of the weekly notified cases for two periods: 1998–2004 (period 1) and 2005–2006 (period 2).
Laboratory surveillance system
To generate hypotheses on a causative agent responsible for the described increase, we selected all regularly detected pathogens causing diarrhoea in Aragon for the period 1998–2006 from the two laboratory databases. For parasites we defined ‘regularly detected’ as at least 100 positive tests in patients with gastroenteritis as a main symptom between 1998 and 2006 and for bacteria and virus we defined ‘regularly detected’ as having been isolated at least five times in stool samples between 2004 and 2006. Bacteria were grouped to species level. We calculated the proportion of each pathogen detected and the 13-week moving average to describe the seasonal pattern of each pathogen.
Laboratory methods and test request
To detect changes in laboratory surveillance (in methods or physicians' prescription behaviour) that could have influenced reported cases, we examined the techniques and protocols used in the two laboratories for 1998–2006. We described the number of laboratory tests requested by physicians and estimated the percentage of positive tests.
Correlations and modelling
To verify the hypotheses on causative agents, we calculated correlations and coefficients of correlation between the weekly notified cases in the syndromic and laboratory database for the two periods. We included in a multivariate model the pathogens most frequently isolated, those presenting a high correlation (P>0·8) or a significant correlation (P<0·05). To identify the best model, we conducted a backward stepwise linear regression with Stata (Stata Corp., College Station, TX, USA) using the likelihood ratio test.
RESULTS
Syndromic surveillance
From 1998 to 2006 the total number of notified diarrhoea cases was 1 044 888 (annual rate 95/1000 inhabitants per year). The mean number of weekly notified cases was 2233 (range 1274–4105) (Fig. 1). We did not detect either missing values or outliers.
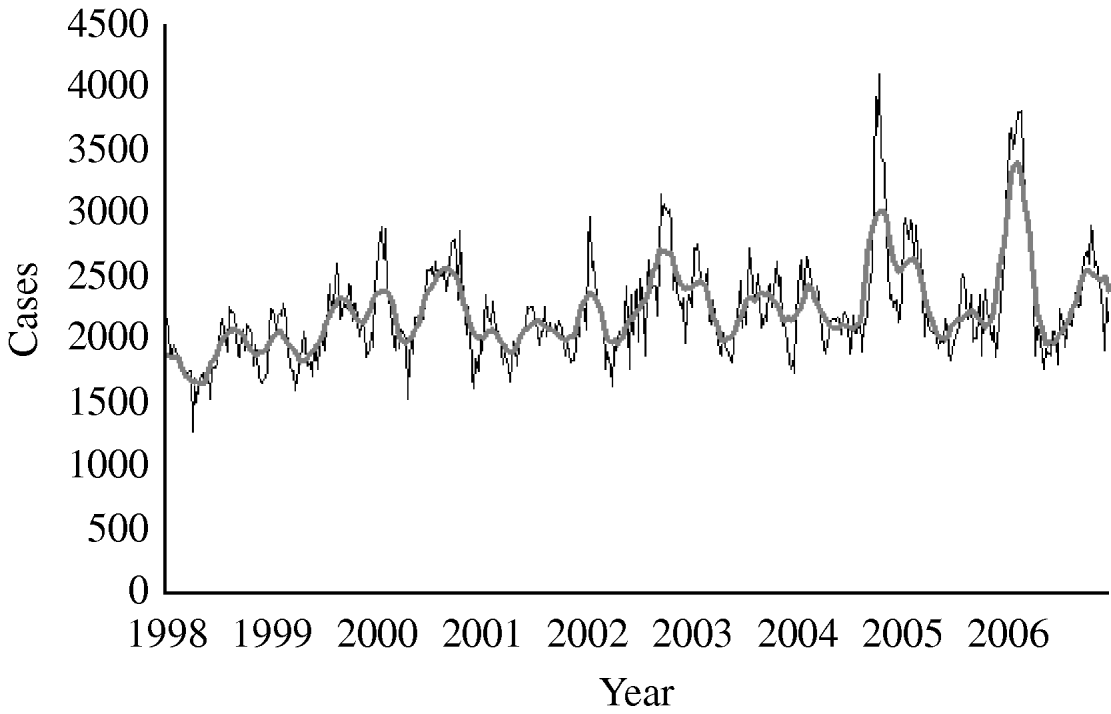
Fig. 1. Number of diarrhoea cases reported by week, 13-week moving average, syndromic surveillance system, Aragon, Spain, 1998–2006. ——, 13-week moving average.
The mean number of weekly cases increased from 2186 in period 1 to 2397 in period 2. In period 1 diarrhoea was most frequently notified in late summer with the highest mean of weekly cases (mean=2681) in week 42 (Fig. 2). In period 2, diarrhoea was most frequently notified in winter. The highest mean of weekly cases was 3357 in week 8 representing an increase of 34·6% compared to period 1 winter (maximum mean 2494). The number of weekly cases during weeks 18–46 was similar to period 1.
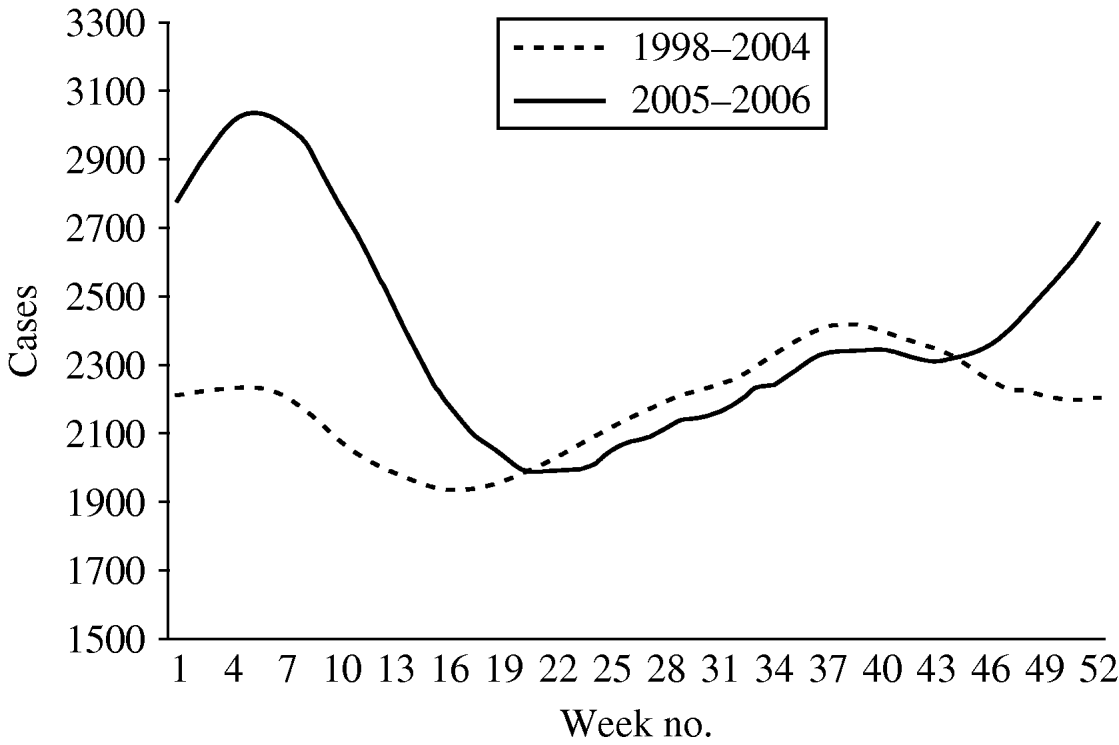
Fig. 2. Mean number of diarrhoea cases reported by week, 13-week moving average, Aragon, Spain, 1998–2006.
Laboratory notification
We selected 11 genus or species of microorganisms out of 310 different diagnoses identified in the laboratory database between 1998 and 2006: Salmonella enterica, Campylobacter spp., Aeromonas spp., Yersinia enterocolitica serogroup O:3, Clostridium difficile, Cryptosporidium spp., Giardia lamblia, Blastocystis hominis, Rotavirus, Adenovirus 40,41 and Astrovirus. Norovirus diagnoses were not performed in Aragon and therefore data for this pathogen were not available. Amongst 5065 pathogens identified, the most frequent in both periods were Campylobacter spp. (29·7%), S. enterica (26·2%) and Rotavirus (17·1%). The mean number of samples positive for Rotavirus per year increased from 22 to 47/100 000 inhabitants per year (268–596 annual tests between the two periods; Fig. 3).
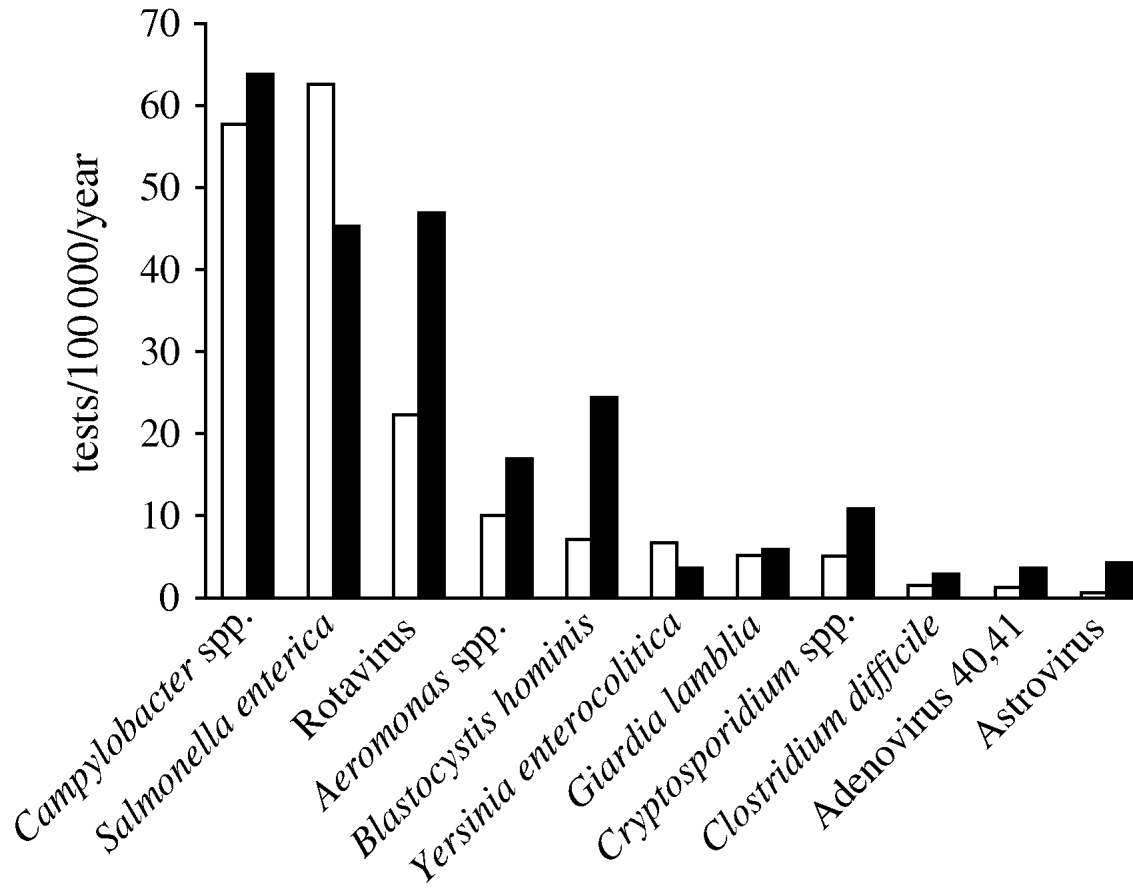
Fig. 3. Mean number of positive tests/100 000 inhabitants per year for 11 pathogens notified by the laboratory during two time periods, Aragon, Spain, 1998–2006. □, 1998–2004; ![]() , 2005–2006.
, 2005–2006.
The weekly number of parasites and bacteria-positive samples did not show a seasonal peak in weeks 1–12 (data not presented). More samples were positive for Rotavirus, Adenovirus 40,41 and Astrovirus in wintertime. A total of 3404 samples were positive for virus. Rotavirus accounted for 90·2% of all positive viral diagnoses and presented a seasonal pattern with more cases reported in winter and a peak of notifications around week 4 (Fig. 4). The number of cases reported per week in weeks 19–40 were similar in the two time periods. In periods 1 and 2 the highest weekly mean of Rotavirus cases occurred around week 4 (mean of 15 and 39, respectively).
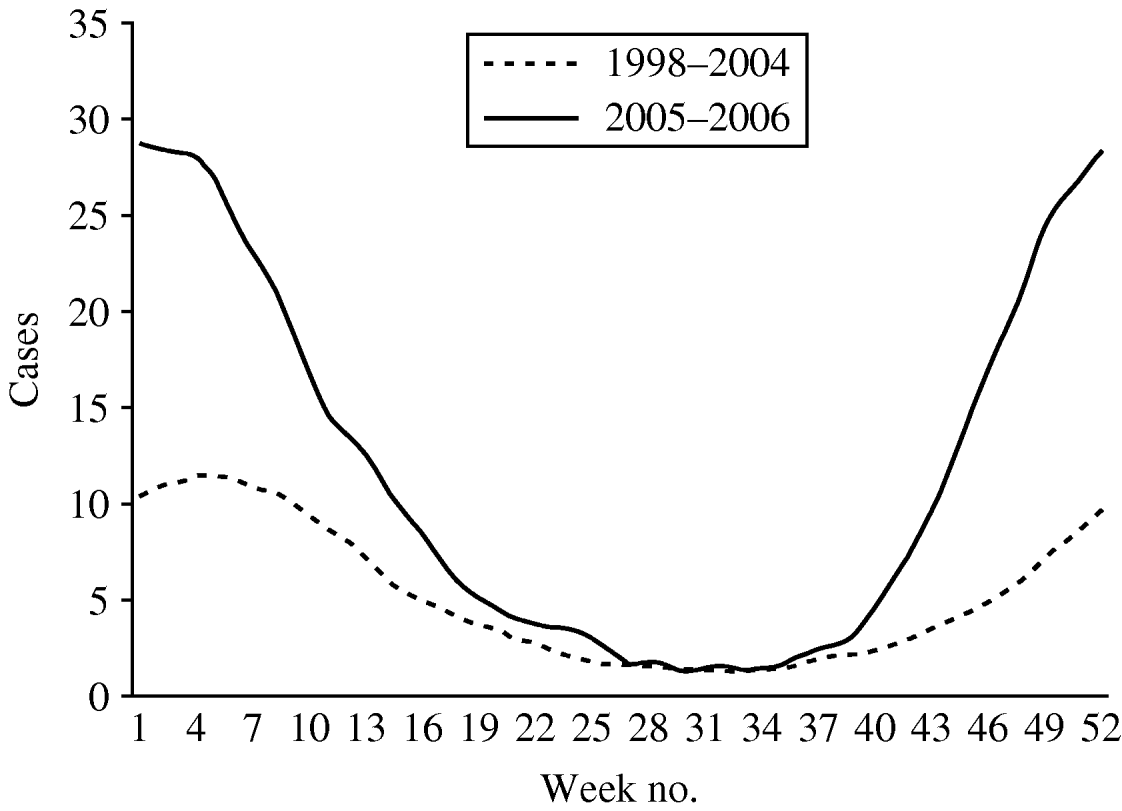
Fig. 4. Mean number of cases of Rotavirus reported by week, 13-week moving average, Aragon, Spain, 1998–2006.
Laboratory methods and tests requested
There were no changes in laboratory techniques in the study period for any of the selected pathogens. Rotavirus was diagnosed using ELISA.
In the two laboratories participating in the laboratory surveillance an annual number of 41 895 tests were requested in 2000–2004 compared to 46 003 in 2005 and 2006, corresponding to an increase of 9·1% (Table 1). An increase was detected for all pathogens in laboratory tests except for Cryptosporidium spp.
Table 1. Annual number of tests requested and number of tests /1000 inhabitants per year in two hospitals in Zaragoza, Spain 2000–2006
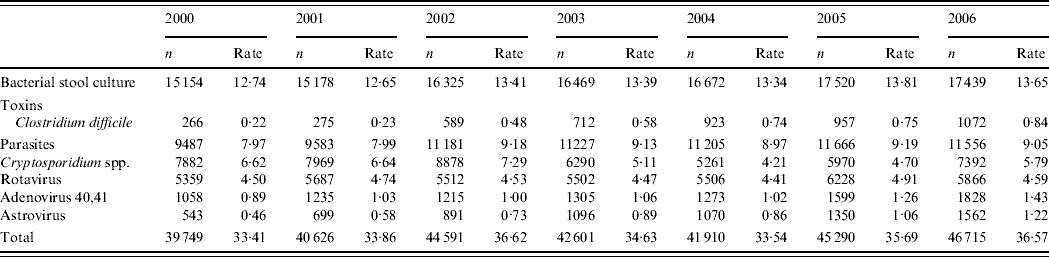
Amongst a total of 37 951 viral tests for Adenovirus 40,41, Astrovirus or Rotavirus requested in 2000–2004, 1467 (3·8%) were reported positive by laboratory surveillance. In 2005 and 2006, 18 433 viral tests were requested among which 1392 (7·6%) were reported positive by laboratory surveillance. Between 2000 and 2006, 1·5% of the tests were positive for Adenovirus 40,41, 1·7% for Astrovirus and 6·3% for Rotavirus.
Correlations and modelling
The correlations between the 11 pathogens and diarrhoea notifications differed between the two time periods (Table 2). In period 1 the highest coefficients of determination were for S. enterica, Cl. difficile, Aeromonas spp., B. hominis and G. lamblia. In period 2 Rotavirus and Astrovirus had the highest coefficients and explained most of the data.
Table 2. Correlations between notifications of diarrhoea from syndromic surveillance and laboratory surveillance in Aragon, Spain 1998–2006
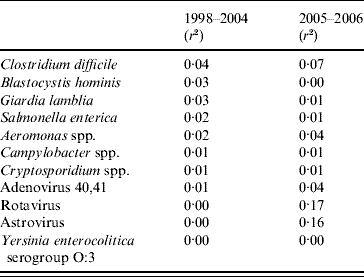
r 2, Coefficient of determination.
The final model for period 1 included S. enterica, Cl. difficile and G. lamblia (Table 3). The model explained 8% of the diarrhoea notifications. In period 2 the model included Rotavirus and Astrovirus, explaining 24% of the diarrhoea notifications.
Table 3. Linear regression model between diarrhoea notifications from the syndromic and laboratory surveillance, Aragon, Spain, 1998–2006
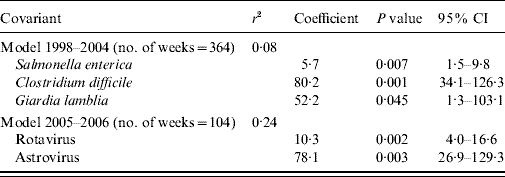
r 2, Coefficient of determination; CI, confidence interval.
DISCUSSION
The number of diarrhoea cases reported in Aragon increased in the winter periods of 2005 and 2006. Our results suggest that Rotavirus could have contributed to this increase. An increase in Rotavirus diagnoses was also reported in the rest of Spain [Reference Sobrino and Soler6], but to our knowledge no recent increase has been reported in other European countries. In Spain in 1994, it was estimated that Rotavirus represented 25·3% of all hospital admissions for gastroenteritis in children aged <5 years [Reference Visser7]. The laboratory tests for Rotavirus did not change over the study period. The increased percentage of tests positive for viral diagnoses suggest that the increase in Rotavirus and other viruses was not the result of an increase in testing, but probably reflects a true increase in the number of cases.
Overall the models can only explain a small part of the diarrhoea cases notified through syndromic surveillance. In studies on syndromic data with respiratory pathogens, higher correlations were found [Reference van den Wijngaard8]. There may be several explanations. First the syndrome of ‘diarrhoea’ is broad and non-specific. Laboratory tests are requested mainly for severe cases that are not representative of the total number of cases. The notified diarrhoea cases might in fact have non-infectious causes or are caused by pathogens that are not regularly tested in the laboratories. The low number of tests for some pathogens may introduce ‘noise’ in the time series and may result in lower correlations.
Limitations
Since there are no media for Norovirus investigation in Aragon, we cannot quantify the role of this virus in the increase of diarrhoea in winter. However, the percentage of positive viral diagnoses increased between the two periods. Therefore we believe that Norovirus was not solely responsible and that other viruses played a role in the increase. In one Spanish study, Norovirus was identified in 31% of the tests among paediatric sporadic diarrhoea cases visiting a hospital [Reference Sanchez-Fauquier9]. However, other studies suggest that Norovirus is less frequent in Spain and that cases occur mainly in summer: in a study in Asturias region, Norovirus was identified in 8·6% of paediatric diarrhoea cases compared to Rotavirus in 36·9%, Campylobacter spp. in 28·8% and S. enterica in 18·4% [Reference Boga10]. In the same study, 50% of all Norovirus cases occurred in June–July with no cases identified in winter. In the United Kingdom peaks of Norovirus were also observed in summertime [Reference Lopman11]. In 2007 Norovirus tests were introduced in some laboratories in Aragon. Until week 30, among 12 positive stool samples of patients with diarrhoea, only one was positive for Norovirus compared to four for Rotavirus [12].
We could only use laboratory notifications from the two main hospitals in the capital of Aragon. Those notifications probably correspond to severe cases of diarrhoea and include mainly samples from diarrhoea cases in the capital. This might be one of the reasons why the models we built could only explain part of the diarrhoea cases included in the syndromic surveillance system.
Surveillance
The current diarrhoeal diseases surveillance system is not enabling timely detection and management of outbreaks. Surveillance of diarrhoeal diseases should be evaluated in Aragon starting with the revision and precise definition of the surveillance objectives in order to identify the most appropriate system(s) to achieve them.
However, combining syndromic and laboratory surveillance was useful to generate hypotheses about the reasons for an increase in cases of diarrhoea in Aragon. The aim of syndromic surveillance is to detect outbreaks earlier than would otherwise be possible with traditional public health methods. It has been used for early detection and follow-up of outbreaks, to monitor disease trends, and to provide reassurance that an outbreak has not occurred [Reference Henning13]. It has been widely used after emergencies [14], in mass-gathering events [Reference Panagiotakos, Costarelli and Polychronopoulos15] or for emerging diseases [Reference Rockx16]. In New York City the evaluation of 3 years of syndromic surveillance based on emergency-department notification, concluded that syndromic surveillance signals are difficult to investigate and should be viewed as a supplement to, rather than a replacement for, traditional surveillance systems that rely on strong ties between clinicians and public health authorities [Reference Balter17]. The syndromic surveillance system in Aragon did not detect alerts in previous years. The specific surveillance system for bacterial foodborne outbreaks may be more readily adapted to identify outbreaks. The syndromic surveillance system aims to follow trends but this objective might be achieved through sentinel surveillance.
Currently there is not a single Rotavirus surveillance system in the various autonomous regions of Spain. Laboratory surveillance and hospitalization records could be useful in surveillance of Rotavirus [Reference Luquero18]. The development of a surveillance system for monitoring the Rotavirus disease burden and the effect of vaccines remains a challenge.
Recommendations and actions taken
In 2007, the Epidemiological Department of Aragon set up a regional laboratory surveillance system including all eight microbiology laboratories in Aragon. In addition, the laboratories of Aragon have introduced Norovirus testing. An enhanced syndromic surveillance was put in place in 2007 that includes clinical and demographic information for each notified case. In 2008 the syndromic surveillance system for diarrhoea in Aragon will be evaluated to assess its usefulness.
Although Rotavirus vaccines are currently not included in the vaccination programme in Spain, the Spanish Paediatric Association advocates informing parents about the existence of the vaccines and their compatibility with vaccines of the vaccination programme [Reference Bernaola19]. We recommend a study to determine the current disease burden of Rotavirus in Aragon and other regions in Spain, using hospital data to have a baseline to evaluate the effect of the availability and use of the vaccines.
ACKNOWLEDGEMENTS
Lisanne Gerstel as a fellow of the European Programme for Intervention Epidemiology Training (EPIET) received a salary financed by the European Commission and the Insituto de Salud Carlos III. We thank the coordinators of the European Programme for Intervention Epidemiology Training (EPIET) and the Spanish Programa de Epidemiología Aplicada de Campo (PEAC) for critical reviews of protocols and drafts. This study was possible thanks to the contribution of colleagues from the Epidemiological Department of Aragon; especial thanks are due to Silvia Martinez, for collecting and providing data and information necessary to perform this study.
DECLARATION OF INTEREST
None.









