INTRODUCTION
Campylobacter is the most commonly reported bacterial cause of infectious intestinal disease in people in the UK and in Europe, and human cases are associated with considerable morbidity and economic loss [1–Reference Roberts5]. As such the reduction of foodborne disease caused by Campylobacter is a key aim of the Food Standards Agency (FSA) strategic plan 2010–2015. In 2009 in England and Wales, 57 772 human cases of campylobacteriosis were reported from diagnostic laboratories to the Health Protection Agency [6]; however, the true burden of infection may be around 400 000 annual cases given that the ratio of Campylobacter cases in the community to reported cases is estimated to be 7·6:1 [Reference Wheeler7]. Most human cases of Campylobacter are considered to be foodborne and the handling or consumption of undercooked chicken is considered to be a major risk factor for human infection [Reference Eberhart-Phillips8–Reference Tam13]. Campylobacter jejuni and C. coli readily colonize the poultry gut and the caecal contents of chickens can contain extremely high numbers of campylobacters (up to 108 c.f.u./g caecal content) [Reference Rosenquist14]. The contamination of poultry carcases with C. jejuni and C. coli occurs during processing and although methods to control this contamination at slaughter are available, they are limited by their practicality, permissibility under European Union (EU) food legislation or acceptability to consumers [Reference Katsma15]. A UK survey of chicken on retail sale found that 65% of fresh chicken samples were Campylobacter positive [16] and more recently 86% of UK carcasses tested were reported as Campylobacter positive in the 2008 EU baseline survey [17]. If the numbers of Campylobacter-colonized flocks and numbers of Campylobacter organisms on carcases were reduced, this may have a major positive impact on public health and become an important part of a key public health strategy in the UK. Indeed, a voluntary industry target has recently been agreed with government in the UK to reduce the percentage of chickens that are most contaminated, measured post-chill at the abattoir and defined as chickens with more than 1000 c.f.u/g [18].
Many risk factors associated with Campylobacter colonization of broiler flocks have been identified. These include the age of the birds at sampling [Reference Barrios19–Reference McDowell21], season [Reference Bouwknegt20–Reference Ellis-Iversen22], production type [Reference Nather23], the presence of other farm animals/cattle on or adjacent to the broiler farm [Reference Bouwknegt20, Reference Ellis-Iversen22, Reference Lyngstad24], partial depopulation/staggered slaughter [Reference Ellis-Iversen22, Reference Hald, Wedderkopp and Madsen25], multiple broiler houses on the farm [Reference Bouwknegt20, Reference McDowell21, Reference Guerin26], flock size [Reference Barrios19, Reference Nather23], water from a private water source [Reference Lyngstad24], drinking water systems [Reference Nather23] and farm hygiene [Reference McDowell21, Reference Hald, Wedderkopp and Madsen25, Reference Evans and Sayers27]. Previous UK studies on the prevalence and risk of Campylobacter in broiler flocks have been published; however, the study populations or study duration have been limited or have excluded older birds or birds that have been previously partially depopulated [Reference Ellis-Iversen22, Reference Evans and Sayers27, Reference Allen28].
A UK-wide abattoir survey was conducted in 2007–2009 and included the EU-wide baseline survey in 2008. The survey examined caecal samples from broiler chickens intended for human consumption. A standardized questionnaire was completed at the time of sampling to provide epidemiological data on the slaughter population. The main aims of the study were to (1) determine the prevalence of Campylobacter in UK broiler batches at slaughter, (2) to investigate the effect of season and other potential risk factors associated with the presence of Campylobacter in broiler batches at slaughter and (3) to provide isolates for sampling validation, enumeration, antimicrobial resistance (AMR) testing and multi-locus sequence typing (MLST). The findings from the survey conducted in 2007–2009, including the observed risk factors are reported in this paper.
METHODS
Survey design
The survey design was consistent with the technical specifications for an EU monitoring scheme for Campylobacter in broilers (Commission Decision 2007/516/EC).
The target sample size was 384 slaughter batches of broilers per annum, based on an expected prevalence of 50% with an accuracy of 5% and a confidence of 95%. It was recommended by the EU that about 10% more than the indicated numbers should be sampled in anticipation of some non-responses and for batches that may not meet the eligibility criteria.
Abattoir recruitment and schedule of sampling
All approved abattoirs processing broilers in Great Britain (GB) during 2006 were contacted by the Centre for Epidemiology and Risk Analysis (CERA), Animal Health and Veterinary Laboratories Agency (AHVLA) and asked to participate in the survey. The recruitment of abattoirs located in Northern Ireland (NI) was carried out by the Veterinary Public Health Unit of the Department of Agriculture and Rural Development (DARD).
The sampling schedule was randomized so that the abattoir, the sampling day and the slaughter batch of birds to be sampled on a given day was based on a random selection. The sampling was also weighted so that the number of slaughter batches to sample per selected abattoir was proportional to the broiler throughput processed by the abattoir. The total number of batches to be sampled was stratified by calendar month; therefore about 37 slaughter batches were scheduled for sampling each month.
The sampling schedule was prepared quarterly and appropriately revised to take into account non-submission of samples and abattoir closures. Where an abattoir ceased trading during the survey, its allocation of samples was rescheduled to other participating plants. No additional abattoirs were recruited.
Sample and data collection
Samples were collected by trained staff of the Meat Hygiene Service (an executive agency of the Food Standards Agency) in GB and by the Veterinary Public Health Unit of DARD in NI.
Full and intact caeca were collected at the time of evisceration. Ten birds were randomly sampled from each slaughter batch avoiding the first birds in the batch to minimize risk of unknowingly including a bird from a different batch. Each pair of caeca was put into a separate plastic pot which was labelled with a unique identifier. After collection, samples were chilled and transported to either the AHVLA at Weybridge (GB abattoirs) or the Agri-Food and Biosciences Institute (AFBI) at Stormont (NI abattoirs) as quickly as possible in an insulated shipping box specifically designed for the survey which maintained the temperature of the contents between +2°C and +8°C for up to 72 h.
A standardized data collection form, labelled with the same unique identifier as the samples was completed by trained personnel after sampling the slaughter batch. Information relating to the flock of origin was collected for each slaughter batch including:
-
• parent company,
-
• abattoir identifier,
-
• farm name and address,
-
• time of collection of birds from farm,
-
• time of arrival of the birds at the abattoir,
-
• time of unloading birds,
-
• time at which processing the slaughter batch began,
-
• time at which processing the slaughter batch ended,
-
• production type (conventional, free-range or organic),
-
• number of birds in slaughter batch,
-
• shed number,
-
• age of birds (days),
-
• average weight of birds (kg),
-
• type of crate used to transport birds to slaughter (open floor, solid floor or both),
-
• flock mortality at 14 days of age (% birds),
-
• flock mortality at 72 h before slaughter (% birds),
-
• rejects from slaughter batch (% birds),
-
• reasons for condemnation (number of birds rejected due to the following conditions: ascites, skin lesions, joint lesions, septicaemic carcase, perihepatitis/peritonitis, pericarditis, emaciated, overscalded carcase, bruised carcase, badly bled carcase and other).
A second standardized questionnaire was sent to the poultry company contact at the abattoir to obtain details on flock depopulation status for the specified slaughter batch as this information was not reliably available at the time of sampling and included:
-
• number of broilers on the holding,
-
• number of chicks placed in the house on day of fill,
-
• date when house was stocked,
-
• date of removal of sampled batch,
-
• date of removal of other batches from the house (including age of the birds, number removed and whether it was partial depopulation or clearance),
-
• depopulation status – whether the sampled slaughter batch was the first batch removed from the house,
-
• thinning – a thinned slaughter batch was defined as originating from a flock which had birds removed from the shed/enclosure ⩾4 days, prior to the sampled slaughter batch.
All of the data and the laboratory results from the slaughter batches sampled were entered onto a specifically designed Microsoft® Access database, collated and analysed at CERA, AHVLA.
Eligibility criteria
Samples had to be tested within 80 h after collection, any batches tested after this deadline were excluded from the analysis. A slaughter batch was defined as a quantity of broilers which had been raised on the same premises or in the same house/shed/range and delivered to the abattoir in the same vehicle. Only slaughter batches consisting of birds from the same flock were eligible.
Microbiological methods
Culture and speciation of Campylobacter from caecal samples were performed by the Bacteriology Department, AHVLA (GB) and by AFBI, Stormont Veterinary Laboratory (NI); methods were harmonized at both sites. The method used for the detection and speciation of Campylobacter spp. in caeca was in accordance with the technical specifications set out in Annex I of the Commission Decision 2007/516/EC, in addition enrichment was carried out. In brief, the caecal contents from ten birds per slaughter batch were aseptically removed and pooled into one composite sample by homogenizing 0·02 g of caecal content from each bird into 2 ml of phosphate buffered saline (PBS), 0·1 m, pH 7·2. The pooled sample was streaked directly onto modified charcoal cefoperazone deoxycholate agar (mCCDA) and additionally inoculated into Exeter enrichment broth. Cultures were incubated in a microaerobic atmosphere at 41·5±1°C for 24–48 h. If typical colonies were not present after 48 h, the corresponding Exeter broth was plated onto mCCDA and incubated as above.
Five suspect Campylobacter colonies were sub-cultured from mCCDA plates on 7% sheep blood agar for confirmation and species identification based on phenotypic methods described in ISO 10272-1:2006(E), this included colony morphology, cell morphology, catalase activity, oxidase activity, hippurate hydrolysis and indoxylacetate hydrolysis. Where possible, five colonies per positive batch were confirmed as Campylobacter spp. and a single colony per positive batch was identified to species level. If at least one colony was identified as Campylobacter spp. from either direct or enrichment cultures, the slaughter batch was recorded as Campylobacter positive.
Analysis
Data were analysed using Microsoft® Excel and Stata v. 10 (StataCorp, USA).
The patterns of seasonality were investigated by fitting periodic regressions to the proportions of positive batches against the month of sampling (1, 2, …, 36) both for the individual species (C. coli and C. jejuni) and overall prevalence. The initial models were of the form:

where Pcpos is the monthly proportion of batches positive. The final models were derived by omitting non-significant terms (P<0·10).
The outcome in the descriptive univariable analysis was a Campylobacter-positive slaughter batch, based on direct culture or enrichment. Continuous variables were recoded as categorical variables according to approximate centile distribution. The exposure ‘recent mortality (%)’ was calculated by subtracting ‘mortality at 14 days (%)’ from ‘mortality at 72 h before slaughter (%)’ as these production parameters were routinely recorded to aid flock management. Univariable logistic regression models were used to screen for potential risk factors and a χ2 test or Wald test statistic using a logistic regression model [29] was used to estimate the statistical significance (P value) of crude associations between exposure variables and Campylobacter status.
Multivariable logistic regression was used to estimate the association of slaughter batch Campylobacter status with various risk factors. Any variable that gave a P value <0·25 at the univariable stage of the analysis were assessed for inclusion in the multivariable model based on the Wald test statistic and likelihood-ratio χ2 test. Variables were entered into the model in a forward stepwise fashion and only variables with P Wald <0·05 were retained [Reference Hosmer and Lemeshow30]. Variables which had more than one level were only retained if one or more level was significantly different from the baseline. A backwards stepwise exclusion of non-significant exposures from the univariable analysis was then performed, using the likelihood-ratio χ2 test to assess for significance and any exposure with a P value >0·05 was removed from the model. The goodness of fit of the model was assessed by the Hosmer–Lemeshow goodness-of-fit test [Reference Hosmer and Lemeshow30]. The modelling approach included the a priori risk factors flock depopulation status, slaughter month (or season) and age of the birds. Other possible confounders were included in the model if they were either (1) associated with the risk factor, (2) biologically meaningful as confounders or (3) if they caused a biologically important change in the odds ratio (OR) of the risk factor when included in the model. All variables in the model were tested for biologically plausible interactions. Dummy variables were created to refine the model and to exclude categories which were not significant. Company was included as a cluster to adjust for multilevel dependencies in the data between slaughter batches from the same poultry company.
The variables were then assessed using a multilevel approach (based on company alone and abattoir alone) to assess whether these other models were a better fit to the data than the logistic regression model obtained. As there was no evidence to suggest the multilevel models were superior to the multivariable logistic regression (P<0·01), the logistic regression model was used with standard errors adjusted for clustering on poultry company and the multivariable results presented here. The model was run twice, first with the variable ‘thinning’ and then with ‘previous partial depopulation’ as the former was nested in the latter. When the exposure thinning was included in the multivariable analysis, it was statistically significant (P<0·001) and had an odds ratio of 11·26 [95% confidence interval (CI) 4·81–26·33]. However, the variable, previous partial depopulation, was included in the final model rather than thinning as more data were available on the former.
RESULTS
Participation
Thirty-three GB abattoirs agreed to participate in the study. These abattoirs processed 86·9% of the total GB slaughter throughput (based on 2007 slaughter data – personal communication, Meat Hygiene Service). In addition, four Northern Irish abattoirs were recruited to the study; these abattoirs processed 99·9% of the total NI slaughter throughput (based on 2007 slaughter data). In total, the abattoirs recruited to this UK study processed 88·3% of the UK slaughter throughput. During the course of the survey, three abattoirs closed; one in 2007 and two during 2008. Thirty of the 37 recruited UK abattoirs, associated with 21 poultry companies, were randomly selected to sample during 2007–2009; the throughput of these abattoirs ranged from 0·07% (the lowest throughput abattoir) to 11·7% (the highest throughput abattoir) of the total throughput of recruited abattoirs. Of the seven abattoirs not randomly selected for sampling, the throughput ranged from 0·003% to 0·68% of the total throughput of recruited abattoirs.
Study population
During the 3-year study period, 1283 slaughter batches were sampled. Of these slaughter batches 1174 (91·5%) were eligible for inclusion in the survey (374 in 2007, 400 in 2008 and 400 in 2009) and were included in the analysis. Most of the ineligible batches were excluded because they were not available for testing within the 80-h deadline (83·5%, 91/109 batches). The eligible batches originated from 642 UK farms, with 39·4% of the sampled batches originating from farms which were sampled more than once during the survey, the highest number of slaughter batches from a single premise was 11 (two farms).
The number of eligible slaughter batches sampled per month varied from 15 (January 2007) to 39 (June 2009) with an average of 33. The number of batches sampled per parent company ranged from 1 to 252 batches (average 56 batches) and from 1 to 147 batches (average 39 batches) per abattoir. The majority of slaughter batches were sampled at abattoirs in England (76·1%, 893/1174 batches) reflecting the larger proportion of abattoirs in this region with higher throughput, compared to Scotland (5·5%, 65 batches), Wales (8·2%, 96 batches) and Northern Ireland (10·2%, 120 batches). Most of the slaughter batches of birds came from conventionally reared flocks (94·8%, 1110/1171 batches), 44 (3·8%) slaughter batches were from free-range flocks and 17 (1·5%) batches were from organic flocks (Table 1).
Table 1. Univariable analysis: association between exposure variables and Campylobacter status in broiler flocks at slaughter

OR, Odds ratio; CI, confidence interval.
* Defined as >3 days between slaughter batch removed and previous batches.
† Mortality at 72 h before slaughter minus mortality at 14 days.
Prevalence
The overall prevalence of Campylobacter spp. (C. jejuni, C. coli, C. lari) in batches of broilers at slaughter was 79·2% (930/1174 batches, 95% CI 76·8–81·5) with little variation between years (82·1% in 2007, 78·3% in 2008, 77·5% in 2009). Prevalence varied by month (Fig. 1) and was lowest in February (68·3%, 71/104 batches) and highest in August (97·1%, 99/102 batches). C. jejuni was the most frequently identified species (74·8%, 696/930 batches). A quarter of the speciated isolates were identified as C. coli (25·1%, 233/930 batches) and one batch, identified in February 2009, was speciated as C. lari (0·1%). A difference in the seasonal distribution of Campylobacter spp. was observed, C. coli appeared to be more prevalent in June, July, August and September compared to winter and spring (Fig. 1).
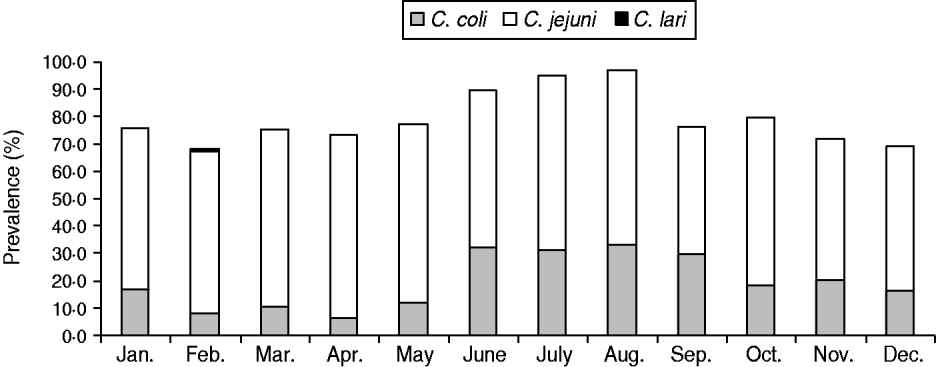
Fig. 1. Campylobacter prevalence and seasonal distribution of Campylobacter spp.
A seasonality model investigating overall prevalence of Campylobacter spp, included two periodic terms. Both regression coefficients were highly significant (P<0·005) indicating a strong seasonal pattern (Fig. 2); the peak months were consistently July and August. A linear trend term over months was not significant when added to this final model (P=0·162). For C. jejuni prevalence, there appeared to be a downward trend over time and therefore a trend term was added to the model. However, once this term was included there was weak evidence to suggest a seasonal pattern and downward trend in C. jejuni, and the model was a poor fit (Fig. 3). However, a strong seasonal pattern in the summer months was observed for C. coli over the 3-year period. Figure 4 shows the predicted and actual proportions of positive samples over the 3-year period. There appeared to be an upward trend in C. coli but when this term was added to the periodic regression model it was not significant (P=0·451).
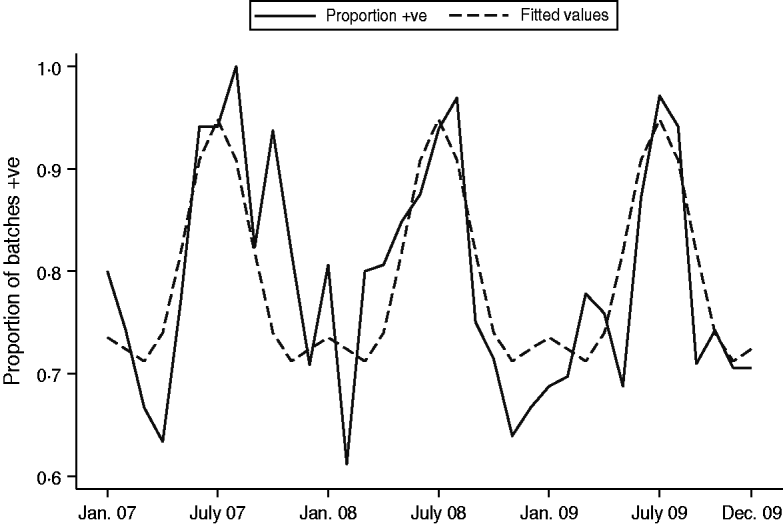
Fig. 2. Seasonality of Campylobacter spp. prevalence: predicted and actual proportions of positive samples over the 3-year period.

Fig. 3. Seasonality of Campylobacter jejuni: predicted and actual proportions of positive samples over the 3-year period.
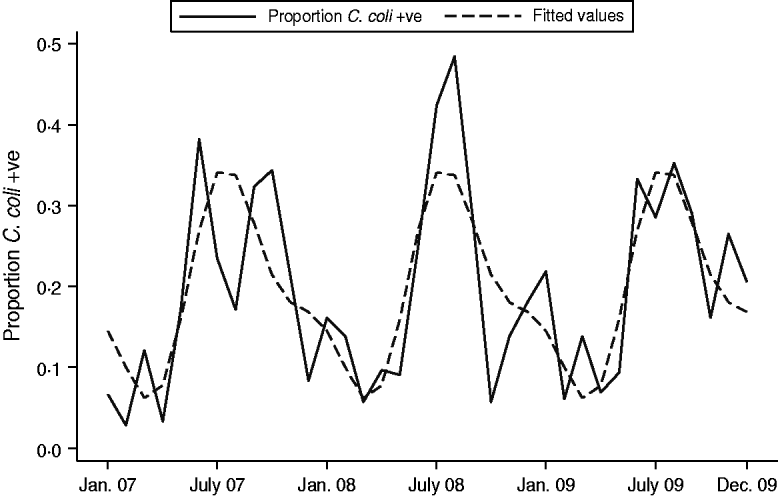
Fig. 4. Seasonality of Campylobacter coli: predicted and actual proportions of positive samples over the 3-year period.
Conventional flocks had the lowest prevalence at 78·5% (871/1110 batches) whereas organic flocks had the highest prevalence with only one batch testing negative (94·1%, 16/17 batches) The prevalence in free-range flocks was 90·9% (40/44 batches).
The age of the birds in the slaughter batch ranged from 27 days to 74 days (average age 41 days). Conventional flocks were typically younger (27–61 days, average 40 days) than free-range or organic birds (free range 48–70 days, average 57 days; organic 64–74 days, average 70 days). The age of the birds was positively associated with Campylobacter prevalence (Table 1). The average age of Campylobacter-positive birds was 42 days (95% CI 41·5–42·4) compared to 37 days (95% CI 36·5–37·9) for Campylobacter-negative birds.
Previous partial depopulation
Data on whether the slaughter batch sampled was from a flock that had previously been partially depopulated was available on 1044 (88·8%) slaughter batches. Of these, 366 (34·1%) batches were the first birds to be removed from the poultry house, the remaining 678 (64·9%) batches were from flocks that had been previously partially depopulated, i.e. other birds had previously been removed from the house for slaughter. The length of time between previous partial depopulation and the sampled slaughter batch varied from 1 to 21 days (average 7 days). The prevalence of Campylobacter in slaughter batches of birds which were the first batch to be removed from the house was 57·9% (212/366 batches, 95% CI 52·7–63·0). In contrast the Campylobacter prevalence in birds that were not the first batch to be removed from the house was 91·2% (618/678 batches, 95% CI 88·8–93·2, P<0·001) (Table 1).
Thinning
To ascertain whether thinning had been carried out the dates of previous removal of birds from the flock were examined. A previously thinned slaughter batch was defined in this study as originating from a flock which had birds removed from it ⩾4 days, prior to the sampled slaughter batch. This information was available for 838 (71·4%) batches and of these 408 (48·7%) batches came from flocks which had been thinned. The prevalence of Campylobacter in previously thinned batches was 96·1% (392/408 batches, 95% CI 93·7–97·7) whereas the prevalence in unthinned slaughter batches was 59·5% (256/430 batches, 95% CI 54·7–64·2, P<0·001).
Risk factors
Twenty-nine variables were tested and 21 showed an association with Campylobacter status during the univariable analysis (Table 1). The final model for Campylobacter-positive flocks included results for 915 slaughter batches and consisted of six variables. Previous depopulation (the removal of birds prior to the sampled slaughter batch), increasing age category, slaughter in the summer months (categorized as June, July and August) or autumn months (categorized as September, October and November), increasing recent flock mortality and increasing time in transit to the abattoir were all included as independent risk factors of Campylobacter-positive batches. Poultry company was a significant variable in the univariable analysis but was not significant when included in the multivariate model. However, once the model was refined the data was clustered by company. An interaction was found between bird age and slaughter in summer and is included in the model. The model outputs are shown in Table 2.
Table 2. Risk factors associated with Campylobacter-positive slaughter batches
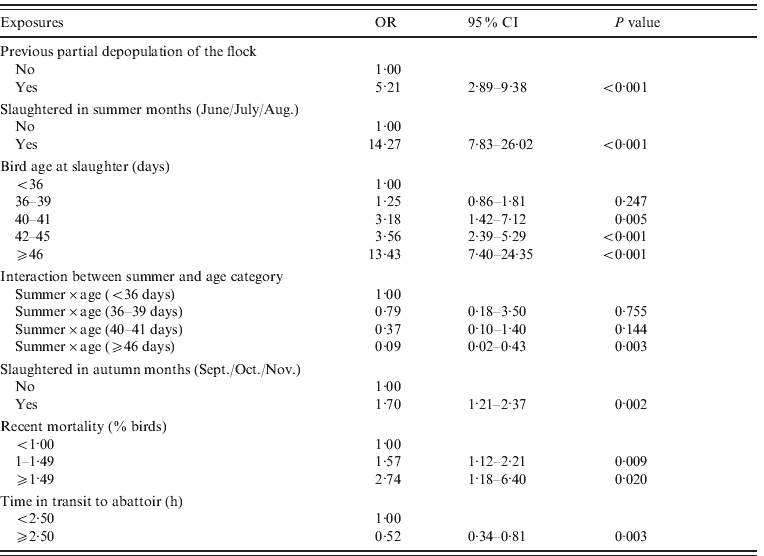
OR, Odds ratio; CI, confidence interval.
DISCUSSION
This is the first UK-wide abattoir survey of Campylobacter in broiler flocks to be performed. The abattoirs participating in the survey processed 88·3% of the UK broiler slaughter throughput; this coverage combined with the randomized sampling approach provides a robust and representative estimate of Campylobacter prevalence and risk factors associated with Campylobacter colonization within the UK broiler population.
During the survey period, 79·2% of the slaughter batches sampled were positive for Campylobacter, this is significantly higher than in UK studies where younger birds/birds prior to the first depopulation were sampled [Reference McDowell21, Reference Ellis-Iversen22] but consistent with an earlier study within GB [Reference Evans and Sayers27]. However, direct comparison of the results between studies is difficult and should be made with caution as both the sampling scheme and isolation method may vary between laboratories and different countries. Indeed one of the aims of the EU baseline survey of Campylobacter in broiler flocks was to achieve harmonized monitoring in order to compare prevalences across Member States. The prevalence observed in our survey, which is harmonized with the EU methodologies, is higher compared to some other European countries, with an average of 71·2% (based on 26 Member States) [17].
C. jejuni was the species most frequently isolated from the caeca (74·8%); however, since the sampling methodology included the speciation of only one isolate per positive slaughter batch, it is possible that some positive batches were colonized with more than one Campylobacter spp. Indeed, additional laboratory work conducted at the AHVLA has shown that birds in 21·6% of the batches tested were colonized with both C. jejuni and C. coli [Reference Rodgers, Randall and Vidal31].
The risk factors identified from the survey included previous depopulation (the removal of birds prior to our sampled slaughter batch), slaughter in summer (categorized as June, July and August) or autumn (categorized as September, October and November), increasing bird age, increasing recent flock mortality and increasing time in transit to the abattoir.
The practice of partial depopulation, whereby a subset of birds of a specific size are selected and removed from the flock and sent for slaughter for economic reasons allowing the rest of the flock to grow, is commonly applied by poultry companies in the UK. This management system has been implicated with increasing the risk for Campylobacter colonization [32–Reference Wedderkopp, Rattenborg and Madsen34], which may be due to the biosecurity problems associated with personnel entering the house for the collection of birds for slaughter and increased stress to the birds, predisposing them to colonization. The risk of colonization was found to be higher in batches where birds had been removed at least 4 days before the sampled batch, this increase in risk may reflect the time needed for Campylobacter to spread and colonize a flock of birds. A potential control measure for reducing Campylobacter in broilers may be to reduce the window between first depopulation and final clearance; however, further work will be needed to be undertaken to address this. It has been argued that the increased risk of Campylobacter from partial depopulation is actually due to the confounding effects of age [Reference Russa35]; however, in our study, previous partial depopulation was still found to be a significant risk factor (OR 5·21) even after adjusting for the confounding effects of age.
The risk associated with the slaughter in summer and autumn months is consistent with other published data [Reference Ellis-Iversen22, Reference Jore36, Reference Kapperud37]. National prevalence studies in other European countries have demonstrated a seasonal peak in Campylobacter-positive flocks, with highest colonization rates occurring in summer or autumn [Reference Barrios19, Reference Bouwknegt20, Reference Jacobs-Reitsma, Bolder and Mulder38, Reference Refregier-Petton39]. The seasonal peak has normally been examined with Campylobacter spp. in general; however, in this study a seasonal variation in the isolation of C. jejuni and C. coli was observed. The seasonal pattern could be due to various environmental factors and indeed, previous studies in the USA, UK and Iceland have suggested that there are associations between environmental factors, such as temperature, humidity, sunlight and low rainfall, and seasonal fluctuations in the carriage of campylobacters by poultry [Reference Doyle40–Reference Arsenault43]. As such the seasonal variation of Campylobacter spp. and the role of climate factors warrants further investigation.
The risk for Campylobacter-colonized broiler batches increased with age of the slaughtered birds. This was an a priori risk factor in our study and is consistent with previous studies in GB and the EU baseline survey [17, Reference Evans and Sayers27]. The interaction between bird age and slaughter in the summer suggests that the increased risk of Campylobacter in older birds (⩾46 days) in the summer months is not as pronounced as in the other seasons. Similarly, the increased risk of Campylobacter in the summer appeared to be most marked in younger birds and less so in the oldest age category. This could be because birds become infected at a younger age in the summer months due to increased load/survival of Campylobacter organisms in the environment (or fomites) associated with higher temperature. The risk of infection is increased and this results in reduced time to colonization. The age association shifts towards younger birds and was not measurable in this study which looked at birds at the end of production, by which time most flocks were colonized. This finding may imply that in the higher age categories, summer is a less significant risk factor for Campylobacter than other factors in the model.
Higher recent flock mortality was found to be significantly associated with an increase risk of Campylobacter infection. However, this variable may be an indicator of flock health and management practices (e.g. poor biosecurity) rather than showing a causal relationship between mortality rate in a flock and Campylobacter infection. A link between bird health and welfare and the Campylobacter status of broiler flocks has previously been reported [Reference Bull44]. Data on husbandry practices or biosecurity at the farm from which the slaughter batch originated were not collected in this study and therefore we cannot attribute mortality data to these or Campylobacter status of the flock, but this finding warrants further investigation.
Increased time in transit to the abattoir was found to be a protective factor when the birds had travelled for 2·5 hours or more. However, this variable is likely to be an indicator of farm size rather than a causal relationship between transit time and Campylobacter infection. Large poultry farms are often situated close to the company abattoir whereas flocks from smaller farms may have to travel further to be slaughtered and as such there may be management and biosecurity differences between large and small farms which were not collected in this study. In a Swedish study, flocks from farms that are situated further away from the slaughterhouse, i.e. ⩾30 km, were also shown to have a reduced risk for Campylobacter [Reference Hansson45].
As the survey was conducted at the abattoir, limited farm information was collected on the sampled slaughter batch. Cattle on or adjacent to the farm and farms with multiple poultry houses have been previously associated with an increased risk of Campylobacter colonization. These exposures were not recorded in our study; therefore, it is plausible that the associations which we have observed may have been confounded by one or more of these farm-level variables.
CONCLUSION
The results reported here are from the first UK-wide Campylobacter survey to be performed in broiler flocks. Campylobacter spp. were isolated from 79·2% of the 1174 slaughter batches. The risk of colonization was highest in the summer months and, to a lesser but still significant degree, in the autumn months and an increased risk was observed with increasing age. Previous partial depopulation and higher recent flock mortality were also identified as risk factors for Campylobacter colonization independent of the strong age and seasonal effects. A longer time in transit to the slaughterhouse was found to be a protective factor. The findings reported here provide a robust estimate of Campylobacter prevalence and risks associated with Campylobacter colonization in the UK broiler population and as such can be used as a representative baseline comparison for future monitoring.
ACKNOWLEDGEMENTS
This project was funded by the Department for Environment, Food and Rural Affairs (project OZ0613), Food Standards Agency (project B15018) and in 2008, EU funding for the mandatory baseline survey. The authors thank the abattoirs and the poultry companies for participating in this study, Agri-Food Biosciences Institute, DARD and the Meat Hygiene Service. They also thank colleagues from the Centre of Epidemiology and Risk Analysis and the Department of Bacteriology for their involvement with the survey. Consultants to the project are thanked for their contribution to the study.
DECLARATION OF INTEREST
None.








