Cryptosporidiosis, caused by infection with the protozoan parasite Cryptosporidium, is a gastroenteric illness that usually presents as voluminous watery diarrhoea and may include other symptoms such as abdominal cramps, vomiting, nausea, fever and loss of appetite. These symptoms can last up to 1 month in healthy individuals with excretion of infectious oocysts continuing for several weeks after symptoms have been resolved [Reference Bouzid1]. Cryptosporidium hominis and C. parvum are responsible for the majority of infections in humans, but C. hominis predominates in human cryptosporidiosis infections in Australia [Reference Ng, MacKenzie and Ryan2, Reference Ng3].
Cryptosporidium is transmitted via the faecal–oral route and is excreted readily infectious in large amounts (105 to 109 oocysts/g stool) with a low infectious dose (10–100 oocysts) [Reference Chappell4]. Cryptosporidium is a significant threat to water utilities as it is able to survive for long periods in the environment and is resistant to drinking-water disinfectants [Reference Bouzid1, Reference Baldursson and Karanis5]. Of the waterborne protozoan parasitic outbreaks that have been reported worldwide between 2004 and 2010, Cryptosporidium was the aetiological agent in 60·3% (n = 120) of the outbreaks [Reference Baldursson and Karanis5].
In November and December 2012, the Kimberley Population Health Unit (KPHU) (located in the Kimberley region of Western Australia) reported 18 notified cases of cryptosporidiosis, compared to the 5-year average for this period of <1 case. The outbreak was suspected to be waterborne as a number of school-aged children that were diagnosed with Cryptosporidium had been swimming at a Broome public swimming pool (BPP). In response to the increase in notifications, the Broome local government environmental health officers (LGEHOs) alerted the local primary school, childcare centres and operators of other swimming pools to advise on any potential risks and infection control.
Molecular tools, such as the glycoprotein 60 (gp60) gene, are widely used to provide sensitive detection and differentiation within and between species of Cryptosporidium, which is impossible by microscopy due to a lack of distinguishing oocyst morphological features [Reference Xiao6]. The gp60 gene is highly polymorphic, enabling individual Cryptosporidium species to be grouped into subtype families and has been widely used in understanding Cryptosporidium transmission dynamics and elucidating sources of oocyst contamination [Reference Xiao6]. Although not routinely performed in Australia, molecular tools were applied to clinical and environmental samples. Environmental and epidemiological investigations were also conducted by the Shire of Broome LGEHOs and OzFoodNet Communicable Disease Control Directorate, respectively, to better understand the source of the outbreak.
For the purposes of the present study, a case was defined as a person diagnosed with cryptosporidiosis with an onset date of illness from November to December 2012 and who was a resident of Broome, Kimberley. Faecal screening for Cryptosporidium was conducted by PathWest Laboratories (Perth) by direct microscopy using a modified Kinyoun's acid-fast stain. As part of routine diagnostic examination, the faecal samples were also tested for Salmonella spp., Campylobacter spp., Shigella spp., Vibrio spp., Yersinia spp., Plesiomonas spp. and rotavirus.
Cryptosporidiosis is a notifiable disease in Western Australia (WA) and cases reported to the Department of Health including basic demographic information of cases (age, race, location and date of onset) were entered into the Western Australia Notifiable Infectious Disease Database (WANIDD). Cases from Broome, Kimberley were referred to the KPHU and interviewed using a structured questionnaire on known risk factors for Cryptosporidium, by the LGEHO), with some assistance from KPHU. Descriptive analysis was conducted based on demographic information of cases and information obtained from the questionnaire survey to identify common exposures.
Where sufficient material was available, faecal samples of the patients that met with the case definition and were diagnosed as microscopy positive for Cryptosporidium by PathWest Laboratories, Perth, were sent to Murdoch University for molecular analysis under Human Ethics approval number 2012/208.
Water samples were collected post-treatment and sent to Murdoch University for molecular analysis on 8 January 2013, in 10-litre aliquots each from three different swimming pool locations; (1) BPP, following reports of additional cryptosporidiosis cases received on 2 January 2013, that indicated that these cases had swum at BPP after superchlorination treatment on 12 December 2012. No further exposure information on these cases was available; (2) a water playground with a small pool located by the beach (WP), which was frequented by children and cases that had been exposed to BPP. Treatment of the pool was by UV radiation; and (3) a swimming pool from a residence in the Roebuck estate, where one case had reported swimming, prior to becoming ill.
The water samples received by Murdoch University were concentrated and purified immediately in accordance with the United States Environmental Protection Agency (USEPA) 1623 method. Briefly, concentration of water samples were carried out using Envirochek filters (Gelman) and resultant eluates were further concentrated for Cryptosporidium by immunomagnetic separation (IMS) using a Dynabeads® GC Combo kit (Dynal Invitrogen, Norway) according to the manufacturer's protocol. The resulting pellet was then subjected to DNA extraction.
Extraction of purified water concentrates and faecal DNA was performed as previously described using a QIAmp DNA Stool kit (Qiagen, Germany) in accordance with the manufacturer's protocol with minor modifications [Reference Ng, MacKenzie and Ryan2]. Molecular characterization of the DNA samples were conducted using a nested PCR and sequence analysis of a fragment of the gp60 gene as described in Ng et al. [Reference Ng, MacKenzie and Ryan2].
A total of 18 patients were diagnosed positive for the presence of Cryptosporidium by microscopy and met with the case definition with an onset date of illness from 9 November to 26 December 2012 (Table 1). These cases were negative for the presence of Salmonella spp., Campylobacter spp., Shigella spp., Vibrio spp., verotoxigenic E. coli, Yersinia spp., Plesiomonas spp. and rotavirus. The average age was 11 years (range <1 year to 47 years). The highest number of cases were from individuals aged 0–4 years (9/18), followed by those aged 5–9 years (5/18). The average duration of diarrhoea was 9 days (range 3–21 days), with no reports of hospitalization. A total of 25 family members of the known cases of cryptosporidiosis were also ill with similar symptoms and with onsets of illness at the same time or after the onset of the notified cases.
Table 1. Details of possible Cryptosporidium exposure of cases and Cryptosporidium species and subtypes identified from cases where molecular genotyping was performed
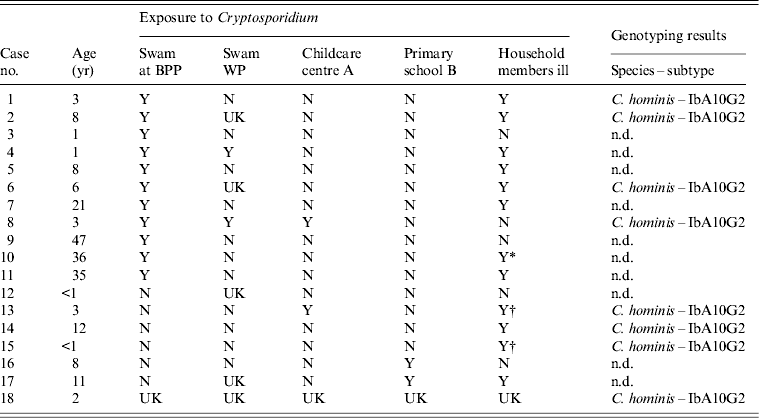
BPP, Broome public swimming pool; WP, water playground; Y, yes; N, no; UK, unknown; n.d., not determined.
* Household member who ill and attends childcare centre A.
† Household member who was ill and attended primary school B.
Eleven of the confirmed cases of cryptosporidiosis (65%) reported swimming at BPP prior to illness onset from 9 November to 26 December 2012 (Table 1). Other potential exposures reported for these 11 cases included swimming at WP (n = 2), other siblings in the household that had similar illness and that had also swam at BPP (prior to the cases becoming ill) (n = 3), and having attended childcare centre A (n = 2) (Table 1). For the seven cases (35%) that did not report swimming at BPP, potential exposures include having attended primary school B (n = 2), attended childcare centre A (n = 1) and other siblings in the household that had similar illness prior to the cases becoming ill (n = 4) with one of the siblings having attended primary school B (Table 1). None of the confirmed cases reported contact with farm or wild animals. Only one individual reported travel outside the Broome area (this individual had returned from India and was unwell on 17 November 2012. This individual (case 7) swam at BPP nearly every day, but contrary to the doctor's advice, had continued to swim at BPP while ill. This person had a housemate who was also unwell with undiagnosed gastroenteritis and swam nearly every day at BPP, and also continued to swim while ill. The onset of illness of the housemate was unknown.
Although 18 cases met the case definition, sufficient faecal material was available for only eight cases and was sent for molecular analysis. PCR amplification and nucleotide analysis at the gp60 gene locus identified C. hominis IbA10G2 subtype in all eight faecal DNA samples analysed (Table 1). Of the water samples collected from three different locations and analysed at the gp60 locus, Cryptosporidium was detected in one water sample, collected from the WP. Sequence analysis identified the C. hominis IbA10G2 subtype. No Cryptosporidium was detected in the water samples from BPP and the Roebuck estate household pool. The water samples were collected 3 weeks after treatment of pools at each respective site with BPP being treated by superchlorination (chlorine levels raised to 20 ppm for at least 12·75 h) and the WP treated with ultraviolet light (UV) irradiation. It is not known if the water sample from the household pool from the Roebuck estate was treated despite the recommendation for superchlorination, or if the water sample was collected pre- or post- treatment.
In the present study, the identification of the same C. hominis IbA10G2 subtype in all eight faecal samples genotyped from an outbreak of cryptosporidiosis in the Shire of Broome and from a water sample from a WP suggests that this particular Cryptosporidium subtype was responsible for the outbreak. Previous analysis of Cryptosporidium subtypes from a longitudinal study in Western Australia from 2005 to 2008 (with corresponding demographic data from WANIDD), revealed that the IbA10G2 subtype was uncommon in the Kimberley region [Reference Ng, MacKenzie and Ryan2]. In that study, the IbA10G2 subtype was responsible for only 5% of cryptosporidiosis cases that were typed, with the majority of cases (>80%) attributed to infection with the C. hominis IdA15G1 subtype. Across Australia, the C. hominis IbA10G2 subtype is the most common cause of sporadic human cryptosporidiosis in Victoria and New South Wales, and the third most common cause of sporadic human cryptosporidiosis in Western Australia. It has also been responsible for previous community-wide outbreaks of cryptosporidiosis in Western Australia and South Australia in 2007 and an outbreak in New South Wales in 2009 [Reference Ng3, Reference Waldron7].
From the investigations conducted, BPP was a likely source of infection, with 65% of the cases reported swimming at the pool. As part of the public health action taken as a result of the outbreak, superchlorination of BPP was carried out on two separate occasions with all ablution facilities cleaned and sanitized, and ablution cleaning equipment replaced with new equipment. Despite a potential source of infection for this outbreak, molecular typing of water samples collected from BPP water was negative for Cryptosporidium by PCR. However, the water sample, was collected ~1 month after the first superchlorination treatment. This followed reports of additional cryptosporidiosis cases received that indicated exposure to BPP after the initial superchlorination treatment. Subsequently, a second superchlorination treatment was performed at BPP. Unfortunately, no exposure information was available for these additional cases although the negative Cryptosporidium pool water result suggests that BPP may not be the source of infection on this occasion.
Although 35% (7/18) of cases did not report swimming at BPP, these cases had contact with people with similar illness at the childcare centre and primary school, which suggests that person-to-person transmission was occurring. There were also family mem bers of cases who had similar illness. Cryptosporidium infection can easily be transmitted from person to person, particularly in the absence of good hygiene measures, such as washing of hands with soap and water after using the toilet or changing diapers and before and after handling of food and tending to someone with diarrhoea [Reference Vandenberg8]. One individual (case 13), who was thought to have become infected after swimming at the pool in the Roebuck estate also had other household members with similar illness. Molecular analysis of the water sample collected from this pool showed that the pool was negative for the presence of Cryptosporidium, providing reason to suggest that the case become ill as a result of person-to-person transmission between household members and not due to swimming in a contaminated pool.
The WP facility did test positive for the C. hominis IbA10G2 subtype. This facility was pre-fitted with a UV filter for water disinfection and as a result of this, the Shire of Broome felt that the facility did not pose a risk for Cryptosporidium contamination. The detection of Cryptosporidium at this site does not necessarily indicate that the UV treatment was unsuccessful as the DNA analysis conducted was not a measure of viability (i.e. the positive PCR result may have come from oocysts that had been inactivated by the UV treatment and were no longer viable). Viability testing would require a cell culture viability assay [Reference King9], which was beyond the scope of the present study.
A limitation in this investigation was that no water samples were collected prior to treatment of the swimming pools for molecular analysis. Comparison of water sample analysis before and after treatment would provide stronger evidence linking potential source of outbreak to cases identified as well as enabling observation of the effectiveness of the water treatment used in these circumstances. However, based on the association of the C. hominis IbA10G2 subtype and waterborne cryptosporidiosis outbreaks, its identification in all outbreak case samples in this study where 65% of cases had exposure to, as well as in a water sample suggests that this outbreak was likely to be waterborne and person-to-person transmission. This is in agreement with the outcome of epidemiological investigations conducted by OzFoodNet and the Broome Local Government and Environmental Health staff.
It is well known that accidental faecal releases (AFRs) in pools by infected swimmers can release large numbers of Cryptosporidium oocysts. For example, an infected individual can excrete up to one billion oocysts during an infectious period and 1 ml faeces can contain as many as 5 × 107 Cryptosporidium oocysts [Reference Polgreen10]. If a child has a loose bowel movement of 150 ml into a typical 25 m × 12 m municipal pool of about 450 m3, this would result in an average concentration of about 20 000 oocysts/litre (20/ml). When an area has a large number of swimmers, these swimmers will contribute to the mixing process. The average swimmer ingests ~28–51 ml pool water (children and adults, respectively) [Reference Schets, Schijven and de Roda Husman11]; therefore, a swimmer swallowing just 10 ml water would ingest an average of 200 oocysts, which is well above a dose capable of causing infection (<100 oocysts) [Reference Chappell4]. During this outbreak period, a total of six AFRs were reported.
The earliest date of onset in this outbreak for any case attending BPP was 9 November 2012. This suggests that the individual (case 7), who swam regularly at BPP and had an onset of illness on 17 November, probably did not start the outbreak but may have contributed to crytosporidiosis transmission after this date. It is important that people with cryptosporidiosis exclude themselves from swimming in pools because even when they become asymptomatic they can still shed oocysts [Reference Ajjampur12]. The WA Department of Health recommends that cryptosporidiosis cases abstain from swimming pools for at least 2 weeks after diarrhoea has stopped. Unfortunately, many cases of cryptosporidiosis are undiagnosed and so are not given advice to exclude themselves from swimming pools. Public swimming pools could review their general signage recommending people avoid swimming if they are symptomatic or recently recovered from any form of gastroenteritis.
The present study highlights the importance of rapid implementation of public health measures to help reduce the spread of infection. It also highlights the need for further research to determine if swimming pools are a common source of community-acquired cryptosporidiosis in Australia and also the need for more effective Accidental Faecal Release Management (AFRM) Guidelines and better education of the public to avoid swimming in pools when they have had infectious gastroenteritis. Currently, nitazoxanide (Cryptaz®, Tri-Med Distributors, Australia) is approved for treatment of cryptosporidiosis in children and immunocompetent adults in the USA [Reference Rossignol13], but is not readily available in Australia. However, the role of nitazoxanide to limit duration of shedding of oocysts [Reference Vandenberg8], deserves more attention for its use in outbreaks in Australia.
ACKNOWLEDGEMENTS
We thank Llew Withers from the Department of Health for providing environmental health advice during the outbreak and Western Australia pathology laboratory staff, particularly those from PathWest, for diagnosing Cryptosporidium and referring specimens for molecular typing.
DECLARATION OF INTEREST
None.




